Sterility testing is critical for cultivated meat production, where even minor contamination can lead to costly batch failures. This process ensures no harmful microorganisms disrupt bioreactor operations, safeguarding both product quality and financial viability. With contamination rates averaging 11.2% - and rising to 19.5% for large-scale production - producers face significant challenges in maintaining sterile environments.
Key points include:
- Main Contamination Sources: Personnel, raw materials, and bioreactor operations are common entry points for microbes.
- Testing Methods: Membrane filtration for large volumes, direct inoculation for smaller samples, and bioburden testing during production are widely used.
- Real-Time Monitoring: Tools like dissolved oxygen sensors and off-gas analysis enable early detection of microbial activity.
- Emerging Technologies: AI-driven monitoring, cold plasma sterilisation, and automated imaging systems offer faster and more precise contamination management.
For cultivated meat producers, combining traditional sterility tests with advanced monitoring solutions is essential to reduce risks and improve production efficiency.
Rocker Discover - How to Perform a Sterility Test?
sbb-itb-ffee270
Contamination Sources in Bioreactor Systems
To prevent batch failures in bioreactor systems, it's crucial to identify where contamination originates. Contaminants typically fall into three main categories: microbial, particulate, and endotoxin. Each type presents unique challenges for cultivated meat production, making it essential to develop specific preventative strategies.
Personnel are the primary source of contamination, often introducing contaminants through skin shedding, improper gowning, or poor hand hygiene [4][7]. Even with stringent protocols, simple movements can disrupt airflow, leading to turbulence or stagnant areas where contaminants can accumulate [4][9]. The U.S. Food and Drug Administration highlights the risks involved, stating, "any manual or mechanical manipulation of the sterilised drug, components, containers, or closures prior to or during aseptic assembly poses the risk of contamination and thus necessitates careful control" [4].
Environmental factors also play a significant role. For example, failing to maintain a positive pressure of 10–15 Pascals can allow unfiltered air to enter sterile zones [3][4]. Additionally, issues such as HEPA filter inefficiencies - where particle retention falls below 99.97% - or compromised compressed gas filters can quickly compromise sterility [4].
Raw Material and Cell Line Contamination
Raw materials entering the bioreactor system are a major contamination risk. Unverified ingredients, growth media components, and cell lines (available through specialized B2B marketplaces) can introduce opportunistic pathogens [2]. The nutrient-rich environment of cell culture media is particularly susceptible to contamination, making cultivated meat processes more vulnerable compared to microbial bioprocesses [8].
Heat-sensitive ingredients that cannot undergo autoclaving are especially risky, as they require alternative sterilisation methods like filtration [1][8]. Moreover, the inoculation process itself carries inherent risks. Even when membranes are disinfected with alcohol or procedures are performed near an open flame, there is no absolute guarantee against contamination during cell line introduction [8]. These risks underscore the importance of thorough raw material verification before they are introduced into the system.
Bioreactor Operational Risks
Daily operations within bioreactors present numerous contamination opportunities. Manual sampling is particularly high-risk, as every access point increases the chance of introducing contaminants [1]. Issues such as compromised seals, damaged O-rings, or unsterilised closures further heighten the risk [4][8]. Additionally, transferring materials from lower-classified areas into higher-classified zones without proper decontamination is another critical vulnerability [7].
Maintaining strict environmental controls is non-negotiable. Pressure differentials between cleanroom areas should be monitored continuously, and any unusual changes must be investigated immediately [4]. In Class 100 (ISO 5) critical areas, particle counts for sizes ≥0.5 μm must remain under 3,520 particles per cubic metre during operations [4]. Furthermore, aerosolising disinfectants or 70% isopropyl alcohol near air samplers can increase particulate readings, while condensate on gas filters may cause blockages or encourage microbial growth [4][7].
These operational risks highlight the importance of implementing rigorous sterility testing methods to safeguard bioreactor processes.
Sterility Testing Methods for Bioreactors
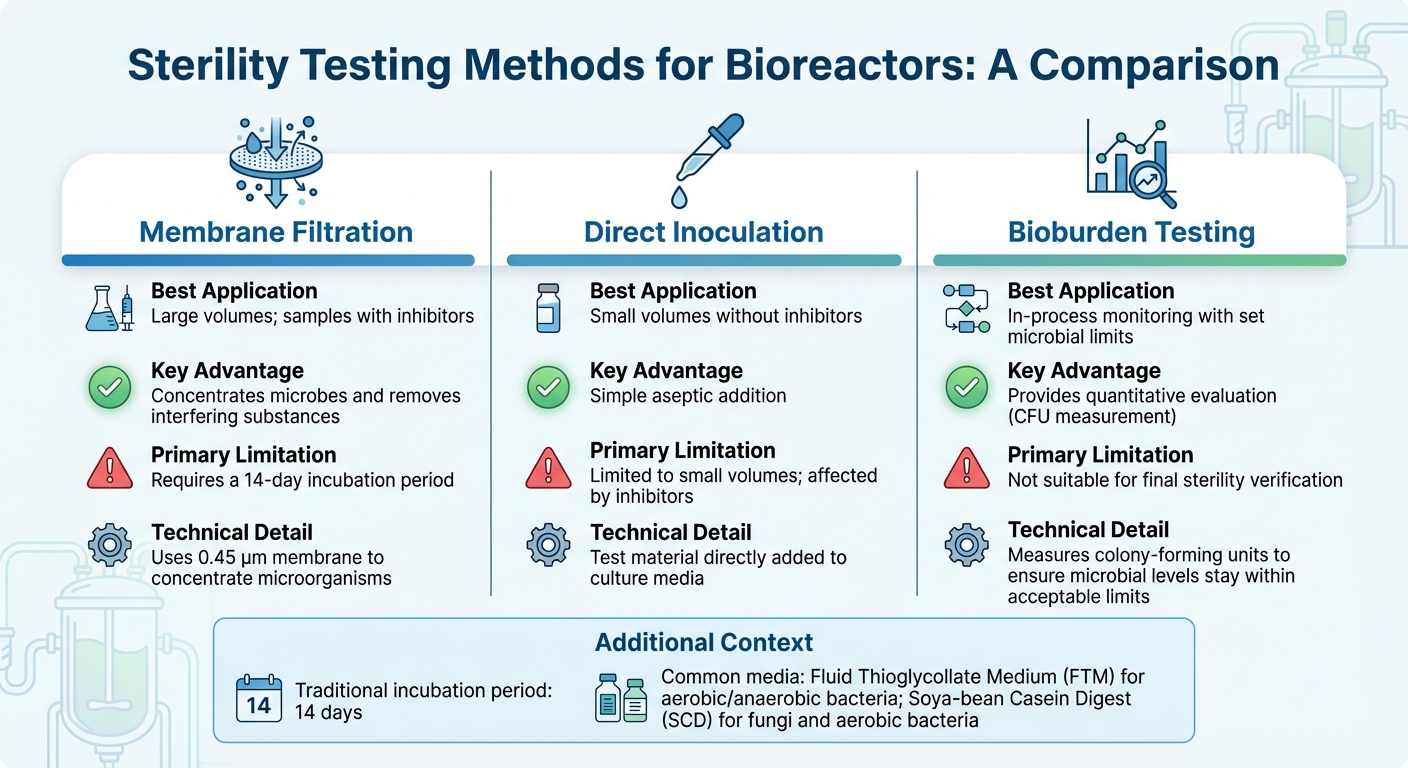
Comparison of Sterility Testing Methods for Bioreactors
Selecting the right sterility test for bioreactors depends on factors like bioreactor size, production stage and scaling challenges, and the sample's composition - especially when inhibitors are present. For most industrial applications, membrane filtration is the go-to method [3]. Meanwhile, molecular techniques like PCR offer quicker detection for specific contaminants. Below, we’ll explore methods tailored to cultivated meat production, addressing the unique challenges of both large and small sample testing.
For large-volume samples, common in industrial-scale bioreactors, membrane filtration employs a 0.45 µm membrane to concentrate microorganisms, improving detection sensitivity [10]. This method is particularly effective for samples containing antibiotics since rinsing can eliminate inhibitors before incubation. On the other hand, direct inoculation, where the test material is directly added to the culture media, works better for smaller sample volumes but struggles to handle inhibitory substances. After concentrating samples and removing inhibitors, incubation and validation ensure the accuracy of results.
Traditional sterility tests rely on a 14-day incubation period to visually confirm microbial growth [3]. Commonly used media include Fluid Thioglycollate Medium (FTM) for aerobic and anaerobic bacteria, and Soya-bean Casein Digest (SCD) for fungi and aerobic bacteria. Before conducting any sterility test, it’s crucial to validate that the product doesn’t inhibit microbial growth through bacteriostasis and fungistasis testing.
For ongoing process monitoring, quantitative bioburden testing offers a more practical solution than binary sterility tests, particularly in cultivated meat production. Unlike sterility tests that provide a simple pass/fail result, bioburden testing measures colony-forming units (CFU) to ensure microbial levels stay within acceptable limits. This method aligns with emerging food-grade standards, striking a balance between stringent pharmaceutical controls and the economic realities of large-scale food production.
For sterility testing supplies and bioreactor solutions, cultivated meat professionals can turn to trusted providers like Cellbase.
| Method | Best Application | Key Advantage | Primary Limitation |
|---|---|---|---|
| Membrane Filtration | Large volumes; samples with inhibitors | Concentrates microbes and removes interfering substances [3] | Requires a 14-day incubation period [3] |
| Direct Inoculation | Small volumes without inhibitors | Simple aseptic addition | Limited to small volumes; affected by inhibitors [3] |
| Bioburden Testing | In-process monitoring with set microbial limits | Provides a quantitative evaluation | Not suitable for final sterility verification [3] |
Real-Time Monitoring and Sterility Assurance
Relying on traditional 14-day sterility tests comes with the risk of losing entire batches if contamination is discovered too late. Real-time monitoring offers a proactive solution by keeping an eye on critical process parameters as they occur. This allows for immediate action if something goes wrong. In cultivated meat production, where bioreactor runs last for weeks and use costly growth media, early detection of contamination can save thousands of pounds and avoid production delays. By combining real-time data with conventional sterility tests, producers can bridge the gap between delayed confirmation and rapid intervention.
Sensor-Based Monitoring
Key indicators like dissolved oxygen (DO) and pH levels can signal contamination early. When bacteria or fungi infiltrate a bioreactor, they quickly consume oxygen - causing DO levels to drop - and release metabolic acids that lower pH significantly [12]. These changes can be detected hours before contamination becomes visually apparent. While traditional sterility tests confirm results after the process, real-time monitoring acts as a safeguard, ensuring the process stays on track and addressing contamination risks earlier.
Off-gas analysis, using magnetic sector mass spectrometry, continuously measures oxygen and carbon dioxide levels in a bioreactor’s exhaust gas. In controlled contamination studies, this method identified microbial growth within 22.4 hours through oxygen changes, while pH-based detection lagged behind at 25.8 hours [13]. Magnetic sector systems deliver precise oxygen measurements with an accuracy of up to 0.003% (v/v) over seven days, outperforming traditional paramagnetic detectors, which are only accurate to ±0.2% (v/v) [13].
Spectroscopic sensors provide non-invasive monitoring through the walls of single-use bioreactors, which is vital for maintaining sterility. UV-vis spectroscopy can detect membrane damage by measuring light absorption at 350–400 nm, while leaked intracellular materials appear at 800–900 nm [14]. Capacitance probes, the only commercially available sensors designed to measure viable cell density, achieve this by detecting changes in membrane polarisation [14]. For facilities managing multiple bioreactors, tools like the Rapid Multi-Stream Sampler can monitor up to 16 gas streams simultaneously [13].
These sensor-based systems, combined with environmental controls, such as HVAC contamination prevention, create a robust defence against contamination.
Environmental and Pressure Controls
Maintaining positive pressure between cleanroom zones is crucial for preventing contaminants from entering [3]. Positive pressure systems, when paired with HEPA filtration, act as physical barriers to microbial intrusion. Audible or visual alarms on HEPA filters can immediately notify staff if pressure drops below acceptable levels [3].
Non-viable particle counting is another layer of defence. Laser particle counters continuously verify that the environment meets ISO air cleanliness standards during operation. By monitoring both 0.5 µm and 5.0 µm particles, these devices ensure air quality remains within required limits [7]. If unexpected deviations occur - such as a sudden drop in DO or a pH fluctuation - immediate isolation of the affected bioreactor and halting feed additions can prevent contamination from spreading [12].
For sourcing specialised sensors and equipment tailored to cultivated meat operations, companies like Cellbase connect professionals with verified suppliers, ensuring access to the right tools for maintaining process integrity.
New Technologies in Sterility Testing
Traditional sterility testing methods often fall short due to their lengthy 14-day incubation periods and reliance on manual sampling, which can leave room for detection gaps. Newer technologies are stepping in to address these challenges, offering faster and more precise contamination detection. This is especially crucial in cultivated meat production, where the high cost of growth media and extended cultivation times make late-stage contamination a financial nightmare.
AI-Driven Monitoring Systems
Artificial intelligence is reshaping contamination detection by analysing real-time data to identify microbial intrusions. When bacteria invade a bioreactor, they consume oxygen and produce metabolic acids, leading to noticeable drops in dissolved oxygen and pH levels. AI systems can detect these deviations in oxygen and nutrient consumption, flagging potential contamination far earlier than traditional bioburden testing and sterility protocols can provide results [12].
These AI platforms also incorporate mathematical models to pinpoint the exact time contamination occurred and simulate how microbial populations grow over time. This helps operators trace contamination back to its source, whether it’s a faulty feed source, operational mishap, or equipment issue. Techniques like Poisson probability analysis further enhance the accuracy of bioburden testing, reducing the likelihood of false negatives [12].
"Mathematical models aid in estimating contamination introduction time and microbial growth dynamics, improving contamination traceability." - Naveenganesh Muralidharan, Senior Manager, MSAT, AGC Biologics [12]
When anomalies are flagged, immediate action - such as isolating the bioreactor and halting all feed additions - can prevent the issue from spreading [12]. A systematic approach involving bioburden testing, molecular identification, and growth rate analysis is essential for identifying the root cause and implementing corrective measures. These AI tools bridge the gap between traditional methods and proactive contamination management.
Cold Plasma Sterilisation
Cold plasma technology offers a non-thermal sterilisation option that’s particularly well-suited for cultivated meat production. Operating at or near room temperature, it’s ideal for sterilising sensitive components like bioreactor parts, sensors, and plastics that can’t withstand the heat of traditional autoclaving [15][16][17]. This method uses reactive oxygen and nitrogen species, along with UV light, to disrupt microbial membranes and DNA. It’s effective against a wide range of contaminants, including bacterial spores (Bacillus, Clostridium), fungi, viruses, and even prions [15][17].
One of cold plasma’s standout features is that it leaves no toxic residues. Once the power is turned off, reactive species quickly revert to oxygen, eliminating the need for a desorption phase [16][18]. It’s also energy-efficient, requiring only a standard electrical outlet instead of fossil-fuel-based heat sources [15][16]. For example, studies show that cold plasma can achieve a >5 log reduction in Bacillus cereus spores within 25 minutes at 300W power [15].
However, the technology isn’t without its limitations. Its sterilisation effects are primarily surface-level, which means it struggles to penetrate complex geometries where microbes may hide in cracks or grooves [15][16]. High protein or fat content in bioreactor environments can also shield microbes by scavenging reactive species, reducing the sterilisation’s efficiency [15][18].
| Feature | Cold Plasma |
|---|---|
| Advantages | Non-thermal, non-toxic, energy-efficient, no residues, fast [16] |
| Limitations | Limited penetration, reduced efficacy in complex geometries [15][16] |
Automated Image-Based Detection Systems
Adding to the mix, automated imaging systems provide a powerful tool for real-time contamination detection. These systems offer detailed morphological insights into cell growth, which is crucial for spotting contamination patterns as they occur [19]. Unlike traditional offline microscopy - which requires manual sampling and staining - automated imaging integrates seamlessly into online or at-line monitoring setups. This allows operators to monitor biomass and cell health without compromising sterility [19].
By reducing manual interventions, these systems cut down on human error and improve reproducibility across cultivation processes [19]. Advanced image-processing algorithms can track fermentation progress, optimise metabolite production, and ensure consistency - a critical factor when scaling up bioprocesses [19].
"The availability of advanced sampling techniques coupled with automated measurement tools... may greatly reduce the time required for strain selection, process development, and process control by diminishing the number of steps in the production/cultivation process, especially manual steps, and cutting down error propagation." - A.C. Veloso and E.C. Ferreira, University of Minho [19]
Despite their advantages, integrating these systems isn’t always straightforward. Cell cultures are complex, raw materials vary, and sensors must withstand harsh sterilisation methods like steam or gamma irradiation [19]. For companies looking to adopt these technologies, platforms like Cellbase can connect them with verified suppliers of imaging systems and sensors tailored for bioprocess applications.
Conclusion
Ensuring bioreactor sterility in cultivated meat production is no small task, but an integrated sterility testing strategy can make it achievable. This strategy blends traditional methods, like membrane filtration for larger sample volumes and direct inoculation for smaller ones, with modern molecular tools such as PCR and qPCR for quick pathogen screening. Additionally, environmental monitoring - via air sampling and surface swabbing - adds an extra safeguard, addressing contamination risks in HVAC systems, catching potential contamination before it can impact production vessels [11].
The choice of testing approach hinges on factors like sample size, the presence of substances that might interfere with results, and whether the focus is on full sterility validation or simply monitoring bioburden. Sampling from multiple points in the bioreactor - top, middle, and bottom - helps create a thorough microbial profile and reduces the chance of missing localised contamination [1]. This is particularly critical given that contamination risks in cultivated meat production are notably higher than in biopharmaceutical manufacturing, highlighting the need for rigorous sterility protocols [6].
Key to maintaining media sterility in bioreactors is sourcing the right equipment. Tools like aseptic sampling systems with pre-sterilised septa and HEPA filters capable of capturing 99.97% of particles larger than 0.3 μm are essential [4]. Platforms such as Cellbase facilitate connections between cultivated meat producers and verified suppliers of sterility testing tools, including membrane filtration units and environmental monitoring equipment tailored to the industry's unique needs.
As the industry grows, hybrid sterility approaches are becoming increasingly important. Applying pharmaceutical-grade controls during early seed train stages, while adopting food-grade standards for large-scale production, strikes a balance between safety and cost-effectiveness [5][6]. These integrated measures will be the cornerstone of safe and efficient cultivated meat production as the field continues to advance.
FAQs
What are the main causes of contamination in bioreactor systems used for cultivated meat production?
Contamination in bioreactor systems happens when the sterile environment is disrupted or when nutrient-rich media provide an ideal setting for microbes to thrive. This can be caused by several factors, such as breaches during sampling, maintenance, or cell harvesting; damaged or blocked gas filters; contamination already present in the growth media; or temporary openings created when installing or servicing sensors. On top of that, worn-out equipment can shed microplastic particles, which may serve as a home for microorganisms.
In the production of cultivated meat, even the smallest contamination can compromise both the safety and the yield of a batch. To reduce these risks, it’s crucial to invest in high-quality equipment like sterile filters, bioreactors, and sensor kits that adhere to strict aseptic standards. Platforms like Cellbase play a key role by connecting manufacturers with dependable suppliers of these specialised tools, helping to maintain sterility at every stage of production.
How does artificial intelligence enhance sterility testing in bioreactors?
AI-powered systems are transforming sterility testing in cultivated meat bioreactors by offering real-time insights through continuous monitoring. Using advanced biosensors, these systems keep track of critical factors like pH, dissolved oxygen, and essential metabolites such as glucose and amino acids. All of this happens without the need for manual checks, which dramatically cuts down the risk of contamination.
What sets these systems apart is their ability to analyse data using algorithms that compare readings to established sterility standards. This means they can detect even the smallest signs of microbial growth far earlier than traditional methods. Beyond just detection, predictive analytics come into play, identifying potential risks like problems during sensor installation or entry through ports. These systems even suggest corrective measures to help producers avoid costly batch losses.
AI-powered microscopy adds another layer of efficiency by instantly distinguishing between healthy cells and contaminants, speeding up sterility verification processes. For producers, platforms like Cellbase make it easier to adopt these advanced biosensors and monitoring tools, ensuring robust sterility testing across operations of all sizes.
What challenges limit the use of cold plasma sterilisation in bioreactors for cultivated meat production?
Cold plasma sterilisation is effective at neutralising microbes, but it comes with a set of challenges when applied to bioreactors in cultivated meat production. One major issue is the limited penetration depth of the reactive species produced by plasma. This makes it tough to sterilise large volumes or densely packed media thoroughly. On top of that, achieving even plasma coverage across an entire reactor becomes increasingly difficult as the system size grows.
Scaling up cold plasma systems from lab settings to commercial-scale bioreactors introduces additional hurdles. Larger reactors demand higher power-to-volume ratios, which can result in sterilisation times that are far from practical. Many cold plasma systems also operate under vacuum conditions or rely on reactive gases, adding layers of complexity in terms of safety, regulatory compliance, and equipment design. These factors make the method less ideal for the large-scale bioreactors typically required in commercial cultivated meat production.
Another concern is the potential for damage caused by reactive oxygen and nitrogen species (RONS), which are key to microbial inactivation. These reactive species can harm sensitive mammalian cells or degrade media components, necessitating careful optimisation to maintain cell viability. As a result, cold plasma is often used in combination with other sterilisation techniques rather than as a stand-alone solution.
Cellbase offers access to a variety of bioreactor platforms and related equipment, giving manufacturers the opportunity to test systems compatible with cold plasma technology in pilot-scale trials.











