Cryopreservation is the process of freezing and storing living cells at ultra-low temperatures to maintain their viability over time. This method is critical for cultivated meat production, ensuring consistent, stable cell lines and protecting against losses from contamination or equipment failure. Key steps include:
- Preparation: Harvest cells during their growth phase, check viability (aim for ≥90%), and prepare them in freezing media with cryoprotectants like DMSO or glycerol.
- Freezing: Use a controlled cooling rate (-1°C to -3°C per minute) to prevent ice crystal damage. Store cells in liquid nitrogen vapour (-135°C to -190°C) for long-term preservation.
- Thawing: Quickly thaw cells in a 37°C water bath to minimise cryoprotectant toxicity, then transfer them to growth media for recovery.
- Quality Control: Label vials accurately, monitor storage conditions, and test viability post-thaw to ensure successful preservation.
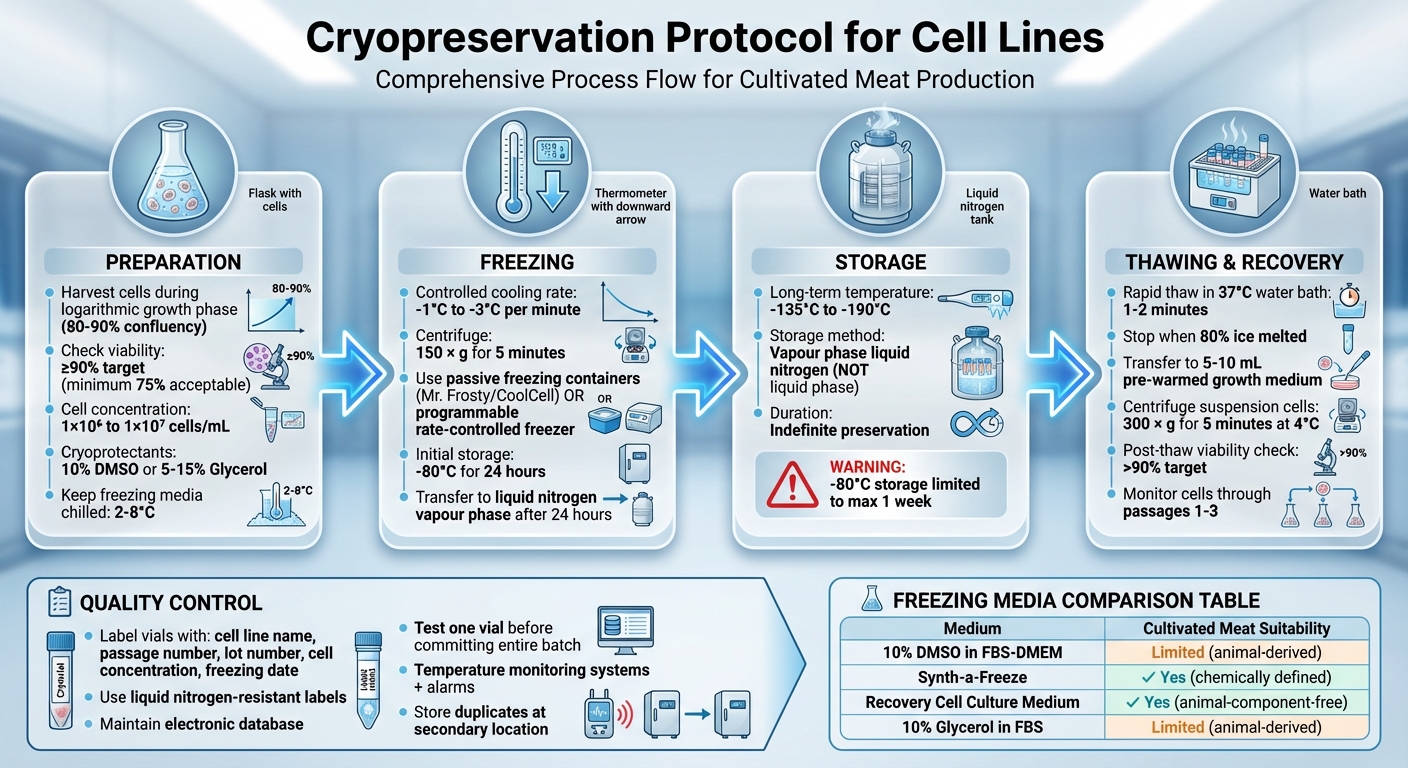
Complete Cryopreservation Protocol for Cell Lines: 4-Step Process from Preparation to Storage
Preparing Cells for Cryopreservation
Cell Harvest and Viability Checks
To ensure the best recovery post-thaw, harvest cells during their logarithmic (log) growth phase. For adherent cell lines, this is typically when they reach 80–90% confluency [2][3][6].
Check the viability of the cells using the Trypan Blue exclusion method. Mix equal parts (1:1) of 0.4% Trypan Blue with the cell suspension, then count the cells using a haemocytometer. Viable cells will exclude the dye and appear bright under the microscope, while non-viable cells will stain blue [4]. Ideally, aim for a viability of at least 90% for the best recovery rates, although some protocols may accept a minimum of 75% [1][2][3][5].
Before harvesting, use a microscope to check for bacterial or fungal contamination. Healthy suspension cells should appear bright, round, and refractile under an inverted phase-contrast microscope [2][3].
Once the cells meet the required viability standards, move on to the pre-freezing steps.
Pre-Freezing Preparations
For adherent cells, use gentle dissociation methods, such as trypsin or TrypLE Express, and limit incubation time to avoid damaging the cell membranes [5]. Prepare the cells at a concentration of 1 × 10⁶ to 1 × 10⁷ cells/mL, depending on the cell line [1][6]. While aliquoting, ensure the cell suspension is mixed frequently to maintain uniform distribution across cryovials [5].
Keep the freezing media chilled between 2°C and 8°C during resuspension to reduce the toxicity of the cryoprotectant before the freezing process begins [5]. Once the cells are suspended in the freezing media, quickly move on to the freezing protocol [1]. Always cryopreserve cells at the lowest possible passage number to reduce the risk of genetic drift or morphological changes [5][7].
Selecting Cryoprotectants and Freezing Media
Cryoprotectant Options and Their Functions
Dimethyl sulfoxide (DMSO) is widely used as a cryoprotectant, typically at a concentration of 10% [2]. It works by penetrating cell membranes and reducing ice formation during freezing. However, DMSO can be toxic to cells at room temperature, so rapid thawing is essential to minimise exposure and quickly dilute it [1].
Glycerol serves as a useful alternative for cell lines sensitive to DMSO, generally used in concentrations ranging from 5% to 15% [8]. It is particularly effective for cell types where DMSO might cause unwanted differentiation [3], and it tends to have lower toxicity compared to DMSO.
In cultivated meat applications, traditional freezing protocols often use a mixture of 90% Fetal Bovine Serum (FBS) and 10% DMSO [1]. However, the reliance on animal-derived components limits these methods in terms of scalability and regulatory approval [9]. To address these issues, chemically defined media - like Synth-a-Freeze or Recovery Cell Culture Medium - provide an animal-component-free alternative. These media maintain high post-thaw cell viability while overcoming the challenges associated with animal-origin components [9].
Comparison of Freezing Media
Here’s a breakdown of the advantages and limitations of various freezing media used in cultivated meat production:
| Medium | Pros | Cons | Cultivated Meat Suitability |
|---|---|---|---|
| 10% DMSO in FBS-DMEM | Established protocols [1] | Contains animal-derived components; batch variability [9] | Limited scalability |
| Synth-a-Freeze | Chemically defined; consistent quality; free of animal components [9] | Higher initial cost [9] | Yes |
| Recovery Cell Culture Medium | Easy to use; designed for rapid recovery [9] | May need optimisation for specific cell lines | Yes |
| 10% Glycerol in FBS | Alternative for DMSO-sensitive cells [1] | Relies on animal-origin serum [9] | Limited scalability |
In February 2023, researchers at Tokyo Women's Medical University, led by Hironobu Takahashi, demonstrated the importance of choosing the right freezing media. Using commercial options like CELLBANKER 1 and 2, they successfully cryopreserved primary bovine myogenic cells at –80°C for up to a year. Remarkably, these cells retained their ability to proliferate and differentiate into contractile muscle tissue with intact sarcomere structures after thawing [10].
For cultivated meat production, chemically defined and GMP-compliant media are increasingly favoured. As STEMCELL Technologies highlights:
In highly regulated fields such as cell and gene therapy, it is recommended to use a GMP-manufactured, fully-defined cryopreservation media to ensure that the products are consistently produced and controlled according to quality standards [9].
Platforms like Cellbase now offer verified, GMP-compliant freezing media specifically tailored to meet the demands of cultivated meat production.
Cryopreservation Procedure and Cooling Rates
Step-by-Step Freezing Protocol
The key to successful cryopreservation lies in maintaining a steady cooling rate of -1°C to -3°C per minute[2]. This gradual process allows water to leave the cells slowly, preventing the formation of damaging intracellular ice crystals that could rupture cell membranes[1].
Begin by centrifuging the cells at 150 x g for 5 minutes[3]. Once centrifuged, resuspend the cell pellet in a cold freezing medium containing 10% DMSO at a concentration of 2–4×10⁶ cells/mL[3]. To reduce DMSO exposure, move quickly to the next step - freezing.
Distribute the cell suspension into pre-labelled cryogenic vials. Each vial should clearly indicate essential details such as the cell line name, passage number, lot number, cell concentration, and the date of freezing[3]. With the vials prepared, it's time to select and utilise the appropriate cooling equipment.
Cooling Equipment and Techniques
Place the vials immediately into a controlled-rate cooling device. Passive freezing containers, like the Nalgene "Mr Frosty" (which uses isopropanol) or the Corning "CoolCell", are popular choices. These tools can achieve a cooling rate of approximately 1°C per minute when placed in a -80°C freezer[2].
For larger-scale operations where consistency is critical, a programmable rate-controlled freezer is the best option. As stated by Sigma-Aldrich:
ECACC routinely use a programmable rate-controlled freezer. This is the most reliable and reproducible way to freeze cells[3].
After around 24 hours at -80°C, transfer the vials to the vapour phase of liquid nitrogen, where temperatures range between -135°C and -190°C, for long-term storage[4]. Avoid storing cells at -80°C for longer than a week, as this can compromise their viability. Temperatures below -135°C are essential for indefinite preservation[2]. Using the vapour phase instead of the liquid phase reduces the risk of cross-contamination while maintaining sufficiently low temperatures.
Thawing and Recovery Protocols
Thawing Process
Thawing cells quickly is crucial to limit toxic cryoprotectant exposure and prevent ice crystals from causing damage. Make sure to wear a full-face visor and insulated gloves for safety. Start by removing the cryovial from liquid nitrogen and slightly loosening the cap to release any built-up pressure. Then, re-tighten the cap.
Place the vial in a 37°C water bath, ensuring the cap stays above the waterline. Let it thaw for 1–2 minutes, or until only a few ice crystals remain. Once thawed, wipe the vial's exterior with 70% alcohol to maintain sterility.
Transfer the contents of the vial into a tube containing 5–10 mL of pre-warmed growth medium. Add the medium slowly to help reduce osmotic shock. If you're working with suspension cell lines, centrifuge the cell suspension immediately at 300 × g for 5 minutes at 4°C. This step helps pellet the cells and removes the cryoprotectant. After centrifugation, resuspend the cells in fresh medium. For adherent cells, centrifugation is usually unnecessary. Instead, directly seed the cells into a suitable culture vessel and remove any residual DMSO during the first media change, typically after 24 hours.
Post-Thaw Assessments
Right after thawing, check cell viability to ensure recovery has been successful. Use the Trypan Blue exclusion method for this assessment. Ideally, cell viability should exceed 90% [11], but a minimum of 75% is acceptable. After 24 hours, inspect the cells under a phase-contrast microscope to confirm adherence, evaluate cell density, and check for any signs of contamination.
Continue monitoring the cells through passages 1–3 to ensure normal proliferation and that they retain their expected characteristics. For cell lines that recover more slowly, you can improve survival by increasing the initial foetal bovine serum concentration to around 20% v/v.
sbb-itb-ffee270
Storage and Long-Term Viability
Storage Conditions and Duration
To maintain cell line viability over the long term, it's essential to store them at temperatures below -135°C [7][2]. This ensures they remain preserved indefinitely.
The preferred method for storing cultivated meat cell lines is vapour phase liquid nitrogen. This technique keeps temperatures between -135°C and -190°C, making it ideal for long-term preservation while offering enhanced safety compared to liquid-phase storage.
If you need to store cells at -80°C, limit this to a period of 24 hours to one week. Beyond this, cell viability may decline. For temporary storage at this temperature, transfer the cells to liquid nitrogen storage as soon as possible.
Use standard sterile cryogenic vials (1–2 mL) with internal threading and an O-ring for safe storage [4][5]. Always place sealed cryovials in the gas phase rather than the liquid phase of nitrogen to reduce the risk of vial explosions during thawing [5]. Additionally, ensure bulk liquid nitrogen vessels are kept at least half full to maintain a safety buffer.
Finally, rigorous quality control measures are critical to ensure the cells' long-term viability.
Quality Control Checks
To ensure the reliability of stored cell lines, follow strict protocols for quality control. Begin by accurately labelling each vial with liquid nitrogen-resistant labels. Include essential details such as the cell line identity, lot number, passage number, and freezing date. Maintain an electronic database to record the exact location of each vial, which reduces the time storage vessels need to remain open [7][2].
Before committing entire batches to long-term storage, test one vial's viability after short-term gas phase storage. This step helps confirm that the freezing process was successful and identifies any potential issues [4][7][2]. For highly valuable cell stocks, it's wise to store duplicates at a secondary location to safeguard against equipment failures or local disasters [7][2].
Equip all storage vessels with temperature monitoring systems and alarms to detect low liquid nitrogen levels [7]. Additionally, install oxygen alarms in storage areas, set to trigger at 18% oxygen (v/v), to minimise asphyxiation risks for personnel working with liquid nitrogen [7][2].
Cryopreservation of mammalian cell lines video protocol
Conclusion and Key Takeaways
Here’s a quick recap of the essential steps and recommendations for effective cryopreservation in cultivated meat production:
- Cell Harvesting: Collect cells during their logarithmic growth phase, ensuring viability exceeds 90%. Use 10% DMSO as the cryoprotectant, though glycerol can be an alternative for more delicate cell lines [11][1].
- Cooling and Storage: Maintain a controlled cooling rate and swiftly transfer vials to vapour-phase liquid nitrogen storage to safeguard cell integrity [11].
A study by Roka Kakehi et al. highlights the importance of precision in cryopreservation [10]:
"Ensuring a reliable and consistent source of cells by using cryopreservation will allow us to increase the stable supply of promising cells for cultivated meat production." - Roka Kakehi et al., Tokyo Women's Medical University
- Thawing Process: Thaw cells in a 37°C water bath for about two minutes, stopping when 80% of the ice has melted. This reduces DMSO toxicity and improves cell recovery [1]. Follow up with post-thaw viability checks to ensure success and fine-tune future procedures.
These methods work hand in hand with strict quality control practices. Always label vials accurately, maintain organised records, and implement thorough checks before long-term storage [11]. For specialised cryopreservation needs, platforms like Cellbase connect researchers with trusted suppliers of controlled-rate freezers, cryogenic storage systems, and other essential tools tailored for cultivated meat production.
FAQs
What are the advantages of using chemically defined media for cryopreserving cell lines in cultivated meat production?
Chemically defined media bring multiple benefits when it comes to cryopreserving cell lines for cultivated meat production. By removing undefined components, like animal-derived serum, they ensure consistent and predictable outcomes - a crucial factor for maintaining the long-term reliability of cell lines.
Another key advantage is the reduced risk of contamination and variability. This not only supports higher quality and safety standards but also aligns perfectly with the precision and scalability required to meet both regulatory demands and consumer expectations in the cultivated meat industry.
How does the choice of cryoprotectant influence cell survival during freezing and thawing?
The choice of cryoprotectant is a key factor in preserving cell health during freezing and thawing. Two widely used options are dimethyl sulfoxide (DMSO) and glycerol, each with distinct characteristics. DMSO is known for its ability to penetrate cells quickly and provide strong protection. However, it comes with a caveat: at high concentrations or with extended exposure, it can become toxic, potentially lowering cell viability.
Glycerol, in contrast, is less toxic and can be applied directly. Its downside lies in its slower rate of cell penetration, which may result in less immediate protection compared to DMSO.
Achieving the right balance is crucial. Properly adjusting the concentration and exposure time of the cryoprotectant helps safeguard cells while minimising the risk of toxicity. Additionally, adhering to best practices for cooling rates and storage conditions is essential to ensure the highest possible recovery rates after thawing.
Why is it important to control the cooling rate during cryopreservation?
Maintaining a steady cooling rate, usually between –1°C and –3°C per minute, is key to keeping cells viable. Cooling gradually allows cells to dehydrate at a controlled pace, reducing the chance of harmful ice crystals forming, which can tear or damage cell membranes.
This measured approach safeguards the cells' structure, boosting their survival and functionality once thawed. Following precise cooling protocols is essential to ensure successful long-term storage and recovery of cell lines.











