Agitation is critical in cultivated meat production, ensuring cells receive oxygen and nutrients while avoiding waste buildup. However, excessive agitation causes problems like cell detachment, membrane damage, and reduced growth. Striking the right balance is essential, particularly in large-scale bioreactors, where even minor adjustments can impact production.
Key Takeaways:
- Optimal Agitation: Studies show 60 rpm in stirred-tank reactors is ideal for balancing nutrient delivery and shear stress.
-
Bioreactor Types:
- Stirred-Tank: Effective mixing but risks high shear stress.
- Wave Bioreactors: Gentle mixing, limited by oxygen transfer.
- Airlift Systems: Uniform mixing with low stress but require precise control.
- Protective Measures: Additives like Poloxamer 188 and bubble-free oxygenation reduce cell damage.
- Scaling Challenges: Larger systems increase shear risks, requiring precise monitoring and CFD modelling.
Maintaining precise agitation control is crucial for scaling cultivated meat production while protecting cell integrity.
How Agitation Affects Cell Growth and Survival
What Recent Studies Show
Recent research has pinpointed specific agitation thresholds that impact cell growth and survival. For instance, an ABM-CFD study using FS-4 cells on microcarriers in a 100 mL stirred-tank bioreactor revealed that 60 rpm is the optimal mixing speed. At this speed, nutrients and oxygen are evenly distributed, with shear stress remaining between 0–80 mPa. However, exceeding 60 rpm leads to cell damage and detachment due to increased forces. At 220 rpm, the impeller Reynolds number skyrockets from 1,444 to 5,294.7, signifying a shift to turbulent flow. This turbulence generates eddies smaller than the microcarriers, which can harm the cells and their membranes [2].
Another study focusing on human umbilical cord-derived mesenchymal stem cells highlighted how even slight increases in agitation intensity significantly reduce adhesion rates. This demonstrates the high sensitivity of adherent cells to mechanical stress [6].
These findings emphasise the importance of precisely calibrating mixing speeds, which remains a key area of ongoing refinement.
Finding the Right Mixing Intensity
The main challenge is balancing the minimum agitation speed needed to suspend microcarriers (N<sub>js</sub>) without crossing shear stress limits. For meat cells, the ideal conditions involve an energy dissipation rate of around 1 mW·kg⁻¹ and a mixing time under 10 seconds [1].
"Maintaining a favourable micro and macro-environment for cells without subjecting them to excessive mechanical stress from stirring will require innovation in and optimisation of bioreactor designs and processes" [2].
Excessive agitation can have two damaging effects: immediate cell death when stress surpasses a critical threshold, and cumulative stress leading to quiescence. Both outcomes hinder productivity. This makes precise control over agitation intensity a critical factor for commercial success, especially in large-scale production. In systems with volumes as large as 20 m³, even minimal agitation can cause cell detachment, highlighting the complexity of scaling up while maintaining cell viability.
Introduction to Bioreactors: Mixing, agitation & shear
Bioreactor Mixing Methods and Their Effects
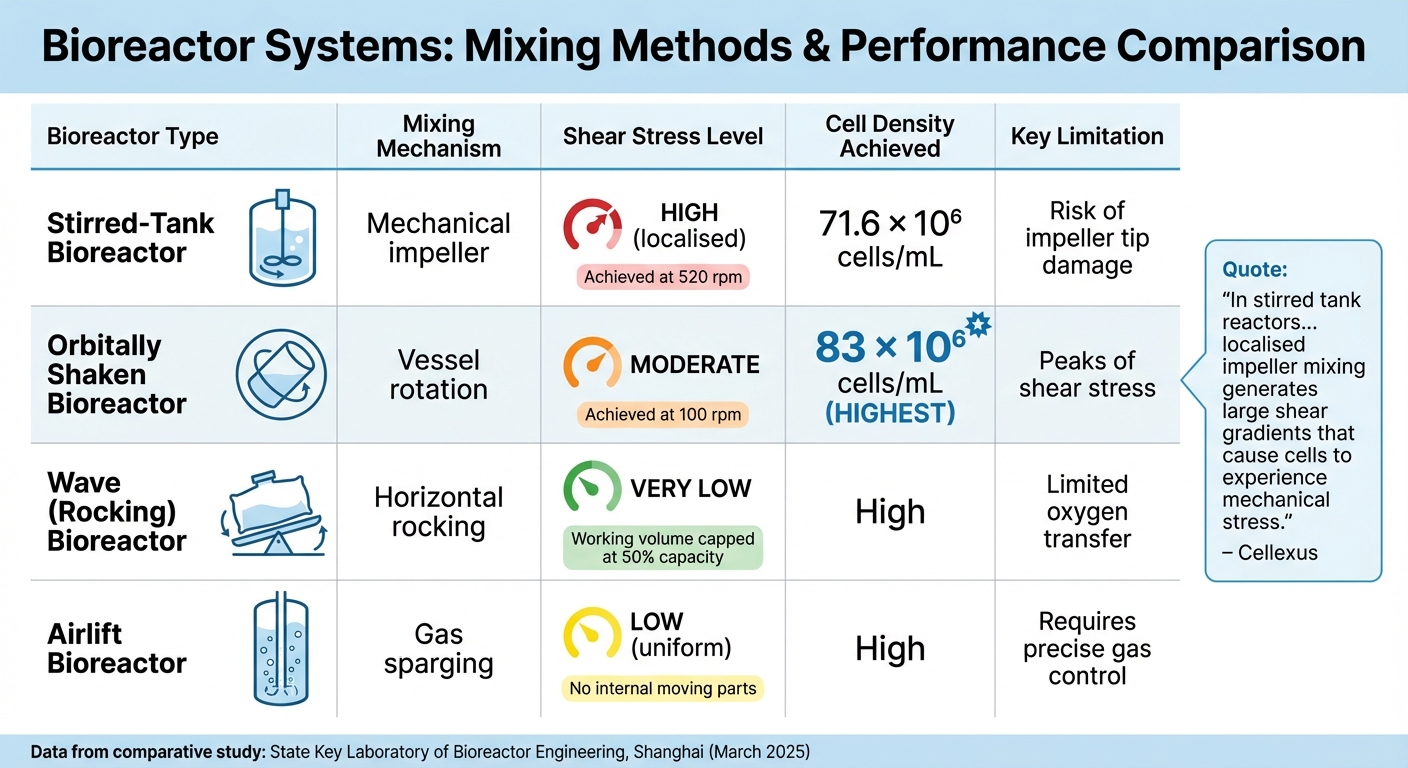
Bioreactor Types Comparison for Cultivated Meat Production
Comparing Different Bioreactor Systems
The design of a bioreactor plays a critical role in balancing nutrient distribution and managing mechanical stress. Each type of bioreactor creates distinct mixing conditions, which directly affect cell survival and productivity. Choosing the right system means finding a balance between efficient nutrient delivery and minimising mechanical forces that could harm the cells.
Stirred-tank bioreactors rely on mechanical impellers to mix the culture. Rushton impellers produce radial flows, leading to localised shear zones, especially near the impeller tips. In contrast, pitched-blade and marine-blade impellers create gentler flows, which are better suited for delicate mammalian cells. A study conducted in March 2025 by the State Key Laboratory of Bioreactor Engineering in Shanghai compared CHO-K1 cell performance in stirred-tank and orbitally shaken bioreactors. The stirred-tank system achieved 71.6 × 10⁶ cells/mL at 520 rpm, while the orbitally shaken system reached 83 × 10⁶ cells/mL at just 100 rpm [4].
Wave (rocking) bioreactors eliminate impellers entirely, using a disposable bag that rocks on a tray to create gentle waves for mixing. This low-shear environment is ideal for fragile cell lines. However, these systems depend on surface aeration, which can limit oxygen transfer in high-density cultures. To maintain effective wave formation, the working volume is capped at 50% of the bag's total capacity [7].
Airlift bioreactors use pneumatic mixing, where gas sparging circulates the liquid between a riser and a downcomer. With no internal moving parts, airlift systems provide uniform energy dissipation and lower shear forces compared to stirred tanks. Unlike wave bioreactors, airlift designs offer better oxygen transfer due to their efficient circulation [7].
| Bioreactor Type | Mixing Mechanism | Shear Stress | Cell Density Achieved | Key Limitation |
|---|---|---|---|---|
| Stirred-Tank | Mechanical impeller | High (localised) | 71.6 × 10⁶ cells/mL | Risk of impeller tip damage |
| Orbitally Shaken | Vessel rotation | Moderate | 83 × 10⁶ cells/mL | Peaks of shear stress |
| Wave (Rocking) | Horizontal rocking | Very low | High | Limited oxygen transfer |
| Airlift | Gas sparging | Low (uniform) | High | Requires precise gas control |
"In stirred tank reactors... localised impeller mixing generates large shear gradients that cause cells to experience mechanical stress." – Cellexus [7]
As bioreactors scale up, the trade-offs between mixing efficiency and cell protection become more apparent. Stirred-tank systems are highly effective at distributing nutrients but require careful speed adjustments to avoid damaging cells in high-shear zones. On the other hand, wave and airlift bioreactors provide gentler mixing, reducing the risk of shear stress, though they may struggle with oxygen delivery in dense cultures. These comparisons underline the delicate balance required to optimise large-scale bioprocessing while protecting cell integrity.
sbb-itb-ffee270
Reducing Shear Stress and Improving Cell Growth
New Bioreactor Designs and Protective Additives
Minimising shear stress is essential for promoting cell growth in cultivated meat production. Innovations in bioreactor design and the use of protective additives have significantly improved cell viability and mixing efficiency. One promising approach involves orbitally shaken bioreactors, which rely on vessel motion and surface aeration to avoid the damaging shear forces caused by impeller-driven mixing and bubble rupture. These systems have demonstrated impressive results, yielding 83 × 10⁶ cells/mL, compared to 71.6 × 10⁶ cells/mL in traditional stirred-tank systems [4].
In stirred-tank systems, the geometry of the impeller also makes a difference. Radial Rushton impellers create flow patterns that allow cells to recover in "calm" zones, reducing the impact of high shear forces. As researchers from TTP observed:
Cells in radial Rushton impeller reactors recover during calm phases, unlike those in double axial impeller systems [5].
For optimal results in cultivated meat production, keeping the impeller-tip velocity within 0.6–1.8 m/s is recommended to protect cell growth [9].
Protective additives like Poloxamer 188 (Pluronic F-68) play a key role by reducing surface tension at the gas–liquid interface, shielding cells from damage during bubble formation and rupture. The ideal concentration for Poloxamer 188 is 1 g/L, as higher amounts provide little additional benefit [9]. For adherent cells grown on microcarriers, an intermittent stirring regime can further enhance outcomes. For example, using a pattern of 30 minutes OFF and 5 minutes ON during the seeding phase encourages bead-to-bead transfer while minimising hydrodynamic stress. This approach has enabled bovine satellite cells to reach densities of 3 × 10⁶ cells/mL [3].
In addition to these design and additive strategies, improving oxygen delivery can further reduce shear stress.
Using Bubble-Free Oxygenation
Bubble-free oxygenation offers another effective way to protect cells from shear damage. Bubble rupture at the gas–liquid interface can generate energy dissipation rates as high as 10⁶ to 10⁸ W/m³, far exceeding the sublethal threshold of 10⁴ W/m³ that most mammalian cells can tolerate [9]. By eliminating bubbles, this method helps safeguard high-density cultures.
Surface aeration, commonly used in orbitally shaken and rocking bioreactors, is particularly effective at reducing shear forces. As highlighted in a recent study:
OSBs utilise vessel body motion and surface aeration to effectively mitigate shear damage caused by traditional impeller blades and bubble formation or breakage [4].
Rocking bioreactors also show promise for cultivated meat production. They offer advantages such as disposability, low operating costs, and a gentle hydrodynamic environment [8].
However, surface aeration does face challenges at very high cell densities. For instance, an orbitally shaken bioreactor achieved an oxygen mass transfer coefficient (kLa) of 20.12 h⁻¹ at 100 rpm, theoretically supporting cell densities up to 118 × 10⁶ cells/mL. In practice, though, as cell density exceeds 80 × 10⁶ cells/mL, the suspension's viscosity increases, leading to non-Newtonian, shear-thinning behaviour that reduces oxygen transfer efficiency. This highlights the need for careful optimisation as cell densities rise.
Controlling Agitation for Large-Scale Production
Adjusting Mixing Speeds and Monitoring Systems
In large-scale systems, maintaining precise control over agitation is crucial. For the first 24 hours, it’s recommended to keep mixing speeds between 30–50 rpm to optimise cell attachment to microcarriers [6]. A study from East China University of Science and Technology in June 2022 highlights the importance of this approach: at 45 rpm, human umbilical cord-derived mesenchymal stem cells achieved a 98.68% adherence rate on Day 1, whereas increasing the speed to 55 rpm caused adherence rates to plummet to 51.32% [6].
After the attachment phase, agitation should slightly surpass the just-suspended speed (N₍JS₎) to prevent cell clumping. Research shows that maintaining an agitation intensity near 1.3 × N₍JS₎ supports cell growth, while exceeding this to 2 × N₍JS₎ hampers growth due to reduced attachment efficiency [10].
Continuous monitoring is critical, given the narrow operational margins. Systems like the BioStar 1.5c bioreactor use advanced software to adjust agitation and gas flow based on real-time feedback from dissolved oxygen (DO) and pH probes [6]. Optical DO sensors play a key role here, offering the precision needed to fine-tune agitation only when DO levels fall below a set threshold - typically around 40% - thereby minimising shear stress [7][6]. The East China team employed this method using Mettler Toledo probes, maintaining DO at 40% and pH at 7.2. This approach resulted in a maximum cell density of 27.3 × 10⁵ cells/mL, a 2.9-fold improvement over standard batch culture techniques [6].
When scaling up, computational fluid dynamics (CFD) models are invaluable for determining the optimal impeller speed to suspend microcarriers without exceeding shear limits [10][6]. Instead of simply matching rpm between vessels, CFD analysis suggests aligning the volume-average shear strain rate between reactors. This ensures that the hydrodynamic environment in a larger bioreactor - such as scaling from a 200 mL spinner flask to a 1.5 L bioreactor - remains conducive to cell growth [6].
These strategies highlight the importance of precise control and monitoring when transitioning to advanced bioreactor systems.
Finding Specialised Equipment Through Cellbase
Sourcing the right equipment for cultivated meat production can be tricky. Standard lab supply platforms often don’t cater to the specific needs of this field, such as low-shear impellers or optical dissolved oxygen sensors tailored for high-density mammalian cell cultures. This is where Cellbase proves invaluable for research and production teams.
As the first dedicated B2B marketplace for the cultivated meat industry, Cellbase connects researchers with trusted suppliers of bioreactor components, monitoring sensors, and microcarrier systems designed specifically for this sector. The platform’s curated listings include detailed specifications - such as compatibility with scaffolds, serum-free systems, or GMP compliance - making it easier to find equipment that meets the exact technical demands of your process. For teams scaling up from spinner flasks to automated bioreactor systems, Cellbase simplifies procurement by offering direct access to suppliers who understand the unique challenges of cultivated meat production. This saves time and reduces the risk of technical mismatches.
Whether you’re upgrading your monitoring systems or sourcing specialised components, platforms like Cellbase streamline the process, ensuring you have the right tools for advancing production.
Conclusion
Getting the balance right between oxygen and nutrient delivery while avoiding harmful shear stress is key to optimising agitation in cultivated meat bioreactors. Research shows this can be achieved by choosing the right bioreactor designs, fine-tuning mixing speeds, and using protective strategies.
Techniques like intermittent stirring, radial Rushton impellers, and real-time adjustments monitored through CFD (Computational Fluid Dynamics) play a big role in ensuring cells recover well and grow steadily. As production scales up from lab flasks to industrial volumes, understanding non-Newtonian fluid behaviour and maintaining consistent Kolmogorov length scales becomes crucial to avoid mechanical damage. These advancements make it easier to protect cells and simplify scaling efforts.
Platforms such as Cellbase further support this process by connecting researchers with suppliers who understand the specific demands of cultivated meat production. This tailored approach helps minimise technical issues and speeds up the journey from small-scale experiments to full-scale commercial operations.
FAQs
What problems can excessive agitation cause in bioreactors for cultivated meat?
Excessive agitation in bioreactors can be a serious problem for cultivated meat production, as it can negatively impact cell growth and survival. Vigorous mixing creates high shear stress, which can harm delicate animal cells. This kind of mechanical stress can result in cell membrane damage, reduced viability, and even hindered tissue development.
To prevent these challenges, it's crucial to fine-tune agitation parameters. The goal is to strike a balance between efficient nutrient and oxygen transfer while minimising mechanical stress. Key factors like impeller design, mixing speed, and the geometry of the bioreactor must be carefully adjusted to maintain healthy, productive cells throughout the cultivation process.
How does the choice of bioreactor affect cell growth and viability in cultivated meat production?
The choice of bioreactor in cultivated meat production is crucial, as it directly impacts cell growth and health by affecting factors like mixing efficiency, oxygen transfer, and shear stress.
Stirred-tank bioreactors are a popular option for large-scale production because they offer precise control over these conditions. However, they can also produce shear forces that might harm fragile cells, making it essential to fine-tune impeller designs and operating parameters to minimise damage.
Other designs, such as airlift bioreactors, are simpler and consume less energy. But they may not provide the same level of control over mixing, potentially affecting cell growth. On the other hand, hollow-fibre bioreactors mimic blood vessels to support high cell densities, though scaling them up can be a challenge.
Selecting the right bioreactor comes down to finding the right balance between factors like scalability, cost, and the specific needs of the cells to ensure they grow and thrive effectively for cultivated meat production.
How can shear stress be reduced during large-scale cultivated meat production?
Minimising shear stress in large-scale cultivated meat production requires careful adjustments to bioreactor design and operation. Factors like impeller type, reactor shape, and mixing settings play a key role. For instance, reducing impeller tip speeds or opting for specific impeller designs can lower shear forces while still maintaining proper mixing and oxygen delivery, which are crucial for cell growth.
Another useful tool in this process is computational fluid dynamics (CFD). CFD simulations enable engineers to study flow patterns and shear distribution in detail, helping them make informed design tweaks. Additionally, rocking or wave-mixed bioreactors offer a gentler alternative to traditional stirred-tank systems, as they naturally produce lower shear forces. Incorporating real-time monitoring with advanced sensors and predictive control algorithms can further help keep shear stress within safe limits, ensuring a smoother production process.











