Cleanroom certification is vital for cultivated meat production, ensuring safety and compliance with UK regulations like Regulation (EC) 853/2004. Without certification, facilities risk contamination, non-compliance, and product quality issues. Here's a quick overview of the process:
- Why Certification Matters: Prevents microbial contamination, aligns with HACCP principles, and ensures consistent production.
- Key Standards: ISO 14644-1 (air cleanliness), EU GMP Annex 1 (sterile manufacturing), and EN 17141 (microbial control).
-
Steps to Certification:
- Design and Build: Install HEPA filters, airflow systems, and maintain proper pressure differentials.
- Installation Qualification (IQ): Verify the cleanroom matches design specs.
- Operational Qualification (OQ): Test performance under controlled conditions.
- Performance Qualification (PQ): Validate functionality during actual operations.
- Continuous Monitoring: Regular checks for particles, pressure, temperature, and humidity.
- Ongoing Compliance: Requalification every 6–12 months or after major changes.
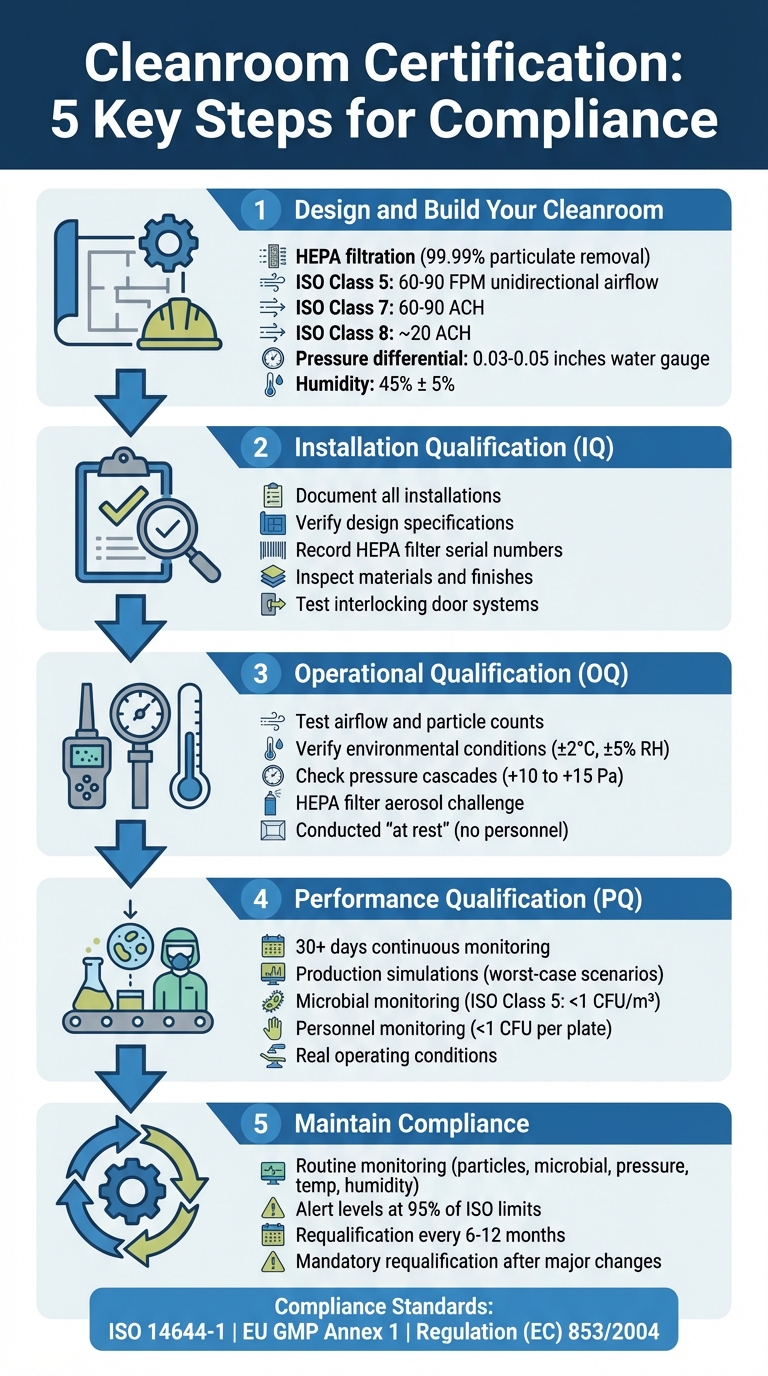
5-Step Cleanroom Certification Process for Cultivated Meat Facilities
Cleanroom Design and Certification
Step 1: Design and Build Your Cleanroom
Building a cleanroom for cultivated meat production requires careful planning around three main systems: HEPA filtration, environmental controls, and workflow separation. These elements ensure sterile conditions and help you avoid costly modifications later. Once these systems are in place, focus on optimising their performance during installation.
HEPA Filtration and Airflow Requirements
The air handling system is the backbone of your cleanroom and determines whether it meets the required ISO classification. HEPA filters remove 99.99% of particulates[5], but their effectiveness depends on delivering air at specific rates and patterns tailored to each production zone.
For ISO Class 5 areas - where aseptic processes like meat harvesting occur - you’ll need unidirectional (laminar) airflow at 60 to 90 feet per minute (FPM)[5]. This involves ceiling-mounted fan filter units (FFUs) directing airflow through low-wall vents with a narrow discharge angle.
In ISO Class 7 and 8 zones, a mixed airflow design is used. Here, fresh supply air blends with internal air, requiring fewer air changes per hour (ACH). ISO Class 7 zones need 60–90 ACH, while ISO Class 8 zones require around 20 ACH[1][6]. To calculate ACH, divide the supply air volume (per hour) by the room volume. Keep in mind that taller ceilings increase both airflow demands and costs[3].
Pressure differentials are key to keeping contaminants out. Maintain a positive pressure differential of 0.03–0.05 inches water gauge between cleanrooms and adjacent areas[7]. However, don’t exceed 0.1 inches water gauge across doors, as this can make them difficult to open - requiring up to 11 pounds of force on a standard 3×7-foot door[7].
After installation, test the filtration system for integrity using aerosol challenge tests like DOP or PAO testing to confirm there are no leaks or weak seals in the HEPA filter housings[1]. Smoke visualisation studies can also help verify laminar airflow and ensure there’s no turbulence or backflow in sensitive zones.
With filtration in place, the next step is to ensure consistent environmental conditions.
Environmental Controls for Production
Maintaining stable temperature, humidity, and pressure is crucial to minimising microbial risks[9]. Your HVAC system must continuously uphold these parameters while supporting the high air change rates required for your cleanroom’s ISO classification.
Temperature control is especially important. The system must counteract heat from bioreactors, lighting, and personnel without disrupting airflow patterns. Relative humidity should remain at 45% ± 5%, which helps prevent electrostatic charges that attract particulates and avoids condensation issues. This range also ensures a comfortable working environment for gowned staff[7].
Real-time monitoring of environmental parameters like pH and dissolved oxygen can help detect early signs of microbial growth[9]. Continuous monitoring of air, surfaces, and water is another essential practice to catch contamination before it escalates. Additionally, design your HVAC system for a short recovery time - this ensures the cleanroom quickly returns to its specified cleanliness level after a contamination event, reducing downtime[1].
Once environmental controls are in place, focus on managing how materials and people move through the space to minimise contamination risks.
Managing Material and Personnel Flow
"Cleanroom workers are a cleanroom's largest contamination source and all critical processes should be isolated from personnel access doors and pathways." - Vincent A. Sakraida, Engineer[7]
Personnel are the biggest contamination risk in cleanrooms, shedding skin particles, hair, and other debris[7][6]. To address this, your cleanroom layout should physically separate critical processes from high-traffic areas and access points.
Materials should move in one direction - from lower-grade zones to higher-grade zones - using validated disinfection or sterilisation processes along the way[8]. Double-ended sterilisers, like double-door autoclaves or depyrogenation tunnels, are ideal for transferring items into aseptic processing areas without compromising air quality[8].
Airlocks act as buffers between areas of different cleanliness levels. ISO Class 7 or cleaner zones should include an anteroom for gowning, which prevents outside contaminants from entering production areas[6]. For Grade A and B zones, interlocking door systems ensure that only one door can be opened at a time[8]. If separate airlocks for personnel and materials aren’t feasible, procedural time-based separation can help avoid simultaneous movement between zones[8].
Limit critical spaces to a single access point to reduce cross-contamination risks[7]. Observation windows or remote cameras can allow supervisors to monitor activities without entering the cleanroom, cutting down unnecessary access[8].
Each of these measures plays a vital role in achieving cleanroom certification and ensuring your facility meets the strict standards required for safe and compliant cultivated meat production.
Step 2: Complete Installation Qualification (IQ)
Once construction wraps up, the next step is Installation Qualification (IQ). This process ensures that every component of the cleanroom has been installed correctly before moving on to operational testing. Essentially, IQ acts as the bridge between completing physical construction and starting HVAC balancing, confirming that everything is ready for the next phase.
"Installation Qualification (IQ) checks ensure that the equipment, components, and cleanroom setup comply with the manufacturer's specifications and that everything has been installed correctly." - Kjeld Lund, Cleanroom Specialist [11]
IQ focuses on the "as-built" state - when the cleanroom structure is finished but production equipment is not yet in place. The main aim here is to ensure that what’s been built matches the original design, with any deviations properly documented and addressed.
Document All Installation Steps
Thorough documentation is key during this phase. You’ll need detailed as-built records, which include updated architectural drawings, lists of HVAC equipment, control sequences, and electrical wiring diagrams. These records should reflect how the cleanroom was actually built, not just how it was planned.
For each HEPA or ULPA filter, record the serial number, exact location, and installation date. It’s critical to inspect filters for transit damage immediately upon installation - since even minor leaks can compromise the cleanroom’s integrity. Tag every piece of equipment and sensor with a unique ID that matches your equipment list, simplifying future audits and maintenance.
Calibration certificates for all monitoring instruments must also be filed. This includes particle counters, differential pressure sensors, temperature and humidity probes, and airflow devices. As Toni Horsfield from ISO Cleanroom explains, "The calibration certificate [for particle counters] is included within your cleanroom validation report." [10]
Inspections of materials and finishes are equally important. Verify that wall panels, flooring, doors, pass-throughs, and sealants meet GMP standards. Surfaces should be non-shedding, low-VOC, and properly sealed. Doors and windows must be flush with walls to maintain pressure integrity.
Keep a deviation log for any design variances, noting the assessments and corrective actions taken. This log will later become part of your final validation report, consolidating all findings from the installation phase.
Verify Design Specifications
Once your installation records are complete, the next step is to ensure that every system aligns with the approved design. Cross-reference the User Requirement Specification (URS) with the physical installations to confirm that nothing was overlooked during shipping or assembly.
For HVAC and filtration systems, check that air handling units, ductwork connections, and diffuser positions match the design drawings. Confirm that HEPA filters are properly seated in their housings and that all ductwork pressure tests have been successfully completed. Record the specifications and data sheets for each fan filter unit.
Structural verification includes inspecting interlocks, airlocks, and pass-throughs to ensure they operate as intended. Test interlocking door systems to ensure that both doors cannot open at the same time. Check that all seals are intact and that the cleanroom can maintain the required pressure differentials.
Run the HVAC system to achieve steady-state conditions before moving on to the next stage of testing.
Conducting IQ with precision is crucial, as it lays the groundwork for all subsequent qualification stages. Skipping steps or rushing through documentation can lead to complications during operational testing and regulatory audits. By completing these checks thoroughly, you ensure a smooth transition to operational qualification.
Step 3: Perform Operational Qualification (OQ)
Once Installation Qualification confirms that everything is installed correctly, the next step is Operational Qualification (OQ). This phase ensures your cleanroom operates as intended under defined conditions. Typically, these tests are conducted "at rest", meaning the HVAC system is running, but no staff or production activities are taking place.
"Validation provides objective proof that the cleanroom does what it was designed to do: maintain a stable, contaminant-free atmosphere under both static (at rest) and operational conditions." - Standard Tech [12]
OQ testing is crucial for demonstrating compliance with ISO 14644-1 and GMP standards. For cultivated meat facilities, this step is especially important since biological processes rely on strict particulate and microbial control. To ensure accurate results, stabilise the cleanroom for at least 30 minutes before starting tests to avoid skewed particle counts [12]. These checks build on the installation phase, laying the groundwork for fine-tuning your environmental controls.
Test Airflow and Particle Counts
Airborne particle counting is the cornerstone of ISO classification. Using a calibrated laser particle counter, measure the concentration of particles in the air to confirm compliance with the required ISO class. For ISO Class 5, refer to the particle limits specified in the standards table.
The number of sampling locations depends on the cleanroom's size. ISO 14644-1 provides clear guidelines: larger rooms require more sampling points, arranged in a grid pattern [16]. If you’re testing two to nine locations, you’ll need to calculate the 95% Upper Confidence Limit (UCL) to determine compliance. For ten or more sampling points, this calculation is unnecessary [15].
Airflow velocity and volume measurements ensure that your air change rate meets design specifications. Use an anemometer to measure velocity at various points, especially near critical process areas, and confirm that these values match your design targets.
Smoke studies offer a visual way to check airflow direction, ensuring it moves from cleaner to less clean zones. Generate smoke near doors, pass-throughs, and other vulnerable areas to detect leaks or turbulence that could disrupt airflow [12]. While particle counters are precise, smoke studies can reveal issues like stagnant zones that might otherwise go unnoticed.
HEPA and ULPA filters must also be revalidated during OQ. Use an aerosol challenge to check for leaks in the filters or their seals. Even minor installation errors can compromise performance, so always retest after any maintenance or filter replacement [12].
Once airflow performance is confirmed, the focus shifts to environmental conditions that impact both product quality and operator comfort.
Verify Environmental Conditions
Temperature and humidity play a significant role in maintaining product quality and ensuring a comfortable working environment. For cultivated meat facilities, validation targets are typically ±2°C for temperature and ±5% for relative humidity [12]. Continuous monitoring over at least 24 hours is recommended, as spot checks might miss fluctuations that could affect validation [12].
Cleanrooms for cultivated meat usually maintain a temperature range of 18–22°C and relative humidity between 30–60% [14]. These conditions support cell culture processes while preventing condensation, which could encourage microbial growth. Use calibrated thermal sensors and RH probes placed throughout the cleanroom to identify any variations in conditions.
Pressure cascades are another critical factor. These ensure that air flows from cleaner to less clean areas, reducing contamination risks. Verify pressure differentials - commonly +10 to +15 Pa between adjacent classified rooms - using calibrated gauges. Take measurements at doorways and pass-throughs under steady-state conditions to confirm proper pressure relationships [12].
Recovery time testing measures how quickly the cleanroom returns to compliance after a contamination event. Introduce a controlled particle source, then monitor how long it takes for particle counts to return to baseline. Faster recovery times indicate better airflow design and more effective contamination control [1].
To avoid delays or costly retests, calibrate all instruments immediately before OQ testing. Record all relevant details, including the date, time, location, instrument ID, and environmental conditions for each test. This documentation is essential for your validation report and will be required during regulatory audits [12].
sbb-itb-ffee270
Step 4: Conduct Performance Qualification (PQ)
Performance Qualification (PQ) evaluates your cleanroom's performance under real production conditions, with equipment running and staff actively working [1][12]. Building on Installation and Operational Qualifications, PQ provides confirmation that the cleanroom performs consistently and reliably during actual operations.
"PQ validates the cleanroom's performance under actual operating conditions, including equipment operation and personnel activity." - G-CON [1]
To ensure thorough testing, the PQ phase should include at least 30 days of continuous monitoring. This extended timeframe helps identify variations, such as temperature fluctuations during production cycles or shifts in microbial contamination due to staff movement, which shorter tests might overlook. For cultivated meat facilities, where strict contamination control is critical, PQ offers documented proof that the cleanroom remains compliant during routine operations.
Run Production Simulations
Production simulations should replicate worst-case scenarios. These might include maximum occupancy, simultaneous operation of all equipment, and temporary contamination risks like frequent door openings or intense movement [1][13]. A risk-based approach, such as using Failure Mode and Effects Analysis (FMEA), can help identify sampling locations based on contamination risks, material flow, and high-traffic areas [16].
Microbial monitoring during these simulations is key. Colony-forming units (CFU) should be tracked using both active and passive sampling methods [14][17]. For ISO Class 5 cleanrooms, the action limit for microbial contamination is generally 1 CFU/m³ [14].
Since humans are the largest source of particles in cleanrooms, personnel monitoring is equally important. Gloved fingertip sampling can confirm proper aseptic techniques, with an acceptable limit of less than 1 CFU per plate [17]. Operators should be briefed on gowning and movement protocols beforehand to prevent artificial spikes in contamination levels [12].
Additionally, test how quickly the cleanroom recovers after a controlled particle challenge. Introduce a particle source and measure the time taken for conditions to return to baseline. This process evaluates airflow and the effectiveness of contamination control systems [1][12].
Set Up Environmental Monitoring
After simulation testing, continuous environmental monitoring ensures consistent performance. These systems provide real-time data on critical parameters like airborne particles, microbial contamination, temperature, humidity, and pressure differentials. This is essential for detecting performance changes before they lead to compliance issues [1]. For cultivated meat production, ongoing monitoring is indispensable.
During the qualification phase, conduct microbial air sampling every 1–2 hours in critical zones to confirm effective contamination control [14]. Use Tryptic Soy Agar (TSA) to detect bacteria, incubating samples at 30–35°C for a minimum of three days, and Sabouraud Dextrose Agar (SAB) for fungi and moulds, incubating at 20–25°C for at least seven days [17]. Avoid using aerosol sprays or disinfectants near air samplers during testing. If rotational disinfectants or 70% isopropyl alcohol have been applied, wait at least five minutes before starting air sampling [17].
Establish clear alert and action limits for all monitored parameters. Alert levels signal the need for investigation when values begin to deviate, while action limits demand immediate corrective action if parameters exceed acceptable ranges [14]. Keep detailed records of every measurement, including date, time, location, instrument details, and environmental conditions. This ensures you are always prepared for audits and can demonstrate compliance with ISO 14644 and GMP standards.
Step 5: Maintain Compliance Through Monitoring
Once you've completed Performance Qualification, the work doesn’t stop there. Maintaining compliance requires continuous monitoring and periodic requalification. Cleanroom certification isn’t a one-time milestone - it demands consistent effort to keep your facility in a "state of control." For cultivated meat facilities, this ongoing process ensures you meet both regulatory and operational standards, extending the meticulous practices of the qualification phases into daily operations.
Implement Routine Monitoring
To ensure your cleanroom remains compliant with ISO 14644 and GMP standards, you need to monitor for microbial and particulate contaminants regularly. Key parameters to keep an eye on include:
- Particle counts
- Microbial levels
- Pressure
- Temperature
- Humidity
- Airflow
The frequency of monitoring should align with your cleanroom's classification and a thorough risk assessment. For instance, ISO Class 5 zones often require continuous or hourly particle monitoring during production, while less critical areas might only need daily or weekly checks.
Set alert levels at 95% of ISO limits to catch potential issues early. These levels act as a warning when parameters begin to drift, prompting investigations before they escalate. On the other hand, action limits demand immediate corrective action if parameters exceed acceptable ranges [14].
Another essential part of monitoring is gloved fingertip sampling (GFS). This method ensures personnel maintain proper aseptic techniques. The standard criterion is typically less than 1 CFU per plate [17]. Performing GFS after critical aseptic tasks or at the end of each shift helps identify and address lapses in technique early.
While routine monitoring helps maintain daily control, requalification ensures your cleanroom systems remain effective over the long term.
Schedule Requalification
Requalification should be carried out every 6 to 12 months. However, certain events make requalification mandatory, such as structural renovations, new equipment installations, HEPA filter replacements, or significant HVAC system changes [1][14].
During requalification, many of the tests from the Operational Qualification phase will need to be repeated. These include:
- Airborne particle counting
- HEPA filter integrity testing (proving 99.99% efficiency for particles ≥0.3 microns)
- Airflow velocity measurements
- Pressure differential checks
One particularly critical test is recovery time testing, which measures how quickly the cleanroom returns to its target cleanliness level after a contamination event. This test verifies your HVAC system’s ability to handle stress effectively [1].
Keep a Validation Master Plan (VMP) to document all qualification stages (IQ, OQ, PQ) and your requalification schedule. Ensure that all instruments used for testing - like particle counters and anemometers - are calibrated and have certificates traceable to national standards [1][14]. This ensures accuracy and reliability in your compliance efforts.
Source Cleanroom Equipment for Compliance
Find Verified Suppliers on Cellbase
Once your cleanroom's operational and performance standards are established and validated, the next step is sourcing the right equipment to maintain compliance. For cultivated meat production, this means working with suppliers who understand the unique demands of the industry.
Cellbase serves as a hub, connecting cultivated meat facilities with verified suppliers of critical cleanroom equipment. From HEPA and ULPA filters to airborne particle counters and environmental sensors, the platform offers listings from trusted manufacturers like Particle Measuring Systems, Vaisala, and Shortridge Instruments. Each listing is tagged with specific use-case details, such as GMP-compliant and ISO 14644-1 certified, making it easier to find exactly what you need. For example, HEPA filters are verified to achieve a 99.97% removal efficiency for 0.3 µm particles, while pressure transducers ensure the required 10–15 Pa differential between adjacent zones [4].
Cellbase also simplifies the process of sourcing prefabricated cleanroom PODs that have undergone Factory Acceptance Testing (FAT). These PODs minimise risks and accelerate commissioning, which is essential for keeping your project on track [1]. Such precision in specifications ensures you can confidently procure materials that align with your facility's compliance requirements.
Ensure GMP-Compliant Procurement
After verifying equipment, the procurement process must also meet stringent GMP standards. Cellbase streamlines this by offering direct access to calibration certificates and equipment manuals needed for Installation Qualification (IQ) documentation [1]. This ensures your facility is audit-ready under regulations like EU Directive 2017/1572 and FDA 21 CFR Parts 210/211.
Additionally, the platform prioritises materials that meet GMP requirements. For instance, construction materials such as 316L stainless steel with polished, non-shedding surfaces are highlighted. These materials are resistant to repeated chemical disinfection and support validated Cleaning-in-Place (CIP) and Sterilisation-in-Place (SIP) protocols [4]. By verifying material compatibility at the outset, you can avoid expensive retrofits or requalification cycles later on. This proactive approach helps maintain compliance while saving time and resources.
Conclusion
Key Takeaways
Achieving cleanroom certification is crucial for maintaining product quality and meeting regulatory standards. It starts with designing a cleanroom equipped with HEPA filtration, controlled airflow, and efficient material flow. The process continues with a three-stage qualification approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These stages ensure that all systems operate effectively under actual working conditions.
Certification doesn't end there. Ongoing monitoring of factors like temperature, humidity, pressure, and particle counts is essential to identify any performance issues. Regular revalidation ensures compliance with ISO 14644-1 and GMP standards, creating a solid framework for future improvements.
Next Steps for Your Facility
To align your facility with these standards, consider developing a Validation Master Plan (VMP). This plan integrates qualification processes with daily operational needs, helping you stay ahead of regulatory demands [1]. Additionally, implementing a HACCP-based food safety management system is key. At least one team member should be trained to Level 4 in HACCP principles to ensure compliance [2].
For equipment needs, turn to Cellbase. This platform connects you with GMP-compliant suppliers, offering everything from HEPA filters to calibrated environmental sensors. By sourcing reliable, audit-ready equipment, you can enhance operational efficiency and maintain compliance effortlessly.
FAQs
What are the benefits of cleanroom certification for cultivated meat production?
Cleanroom certification plays a crucial role in cultivated meat production by ensuring compliance with strict safety and environmental standards. Certified cleanrooms are designed to minimise contamination risks from microbes and particles, maintaining the sterile conditions essential for cell cultivation. This not only protects the quality and safety of the final product but also ensures compliance with internationally recognised standards such as ISO classifications and GMP grades - key requirements for regulatory approval and market acceptance.
Beyond compliance, certification enhances operational reliability by validating critical systems like airflow, filtration, and environmental monitoring. These systems work together to reduce contamination risks, enabling consistent production and improving overall process efficiency. A certified cleanroom also instils confidence among stakeholders, simplifies regulatory inspections, and supports scaling efforts by showcasing adherence to best practices in managing controlled environments.
How often should cleanrooms be requalified to ensure compliance?
Cleanrooms need to be requalified regularly to ensure they meet industry standards. How often this happens depends on several factors, including the cleanroom's classification, how it's used, and findings from risk assessments or environmental monitoring plans.
Typically, requalification is done once a year. However, high-risk environments or situations involving major changes - like equipment upgrades or layout adjustments - might call for more frequent checks. Ongoing performance monitoring is also crucial to verify that airflow, filtration, and environmental controls continue to meet the required standards.
What environmental factors need to be monitored in a cleanroom for cultivated meat production?
To ensure compliance and reduce contamination risks in cultivated meat facilities, it's crucial to keep a close eye on several environmental factors. These include particle counts, microbial contamination, airflow patterns, air pressure differentials, temperature, and humidity levels. Regular monitoring of these elements helps maintain GMP standards and creates a controlled environment essential for production.
By carefully managing these conditions, facilities can protect product quality while meeting the strict criteria required for cleanroom certification.











