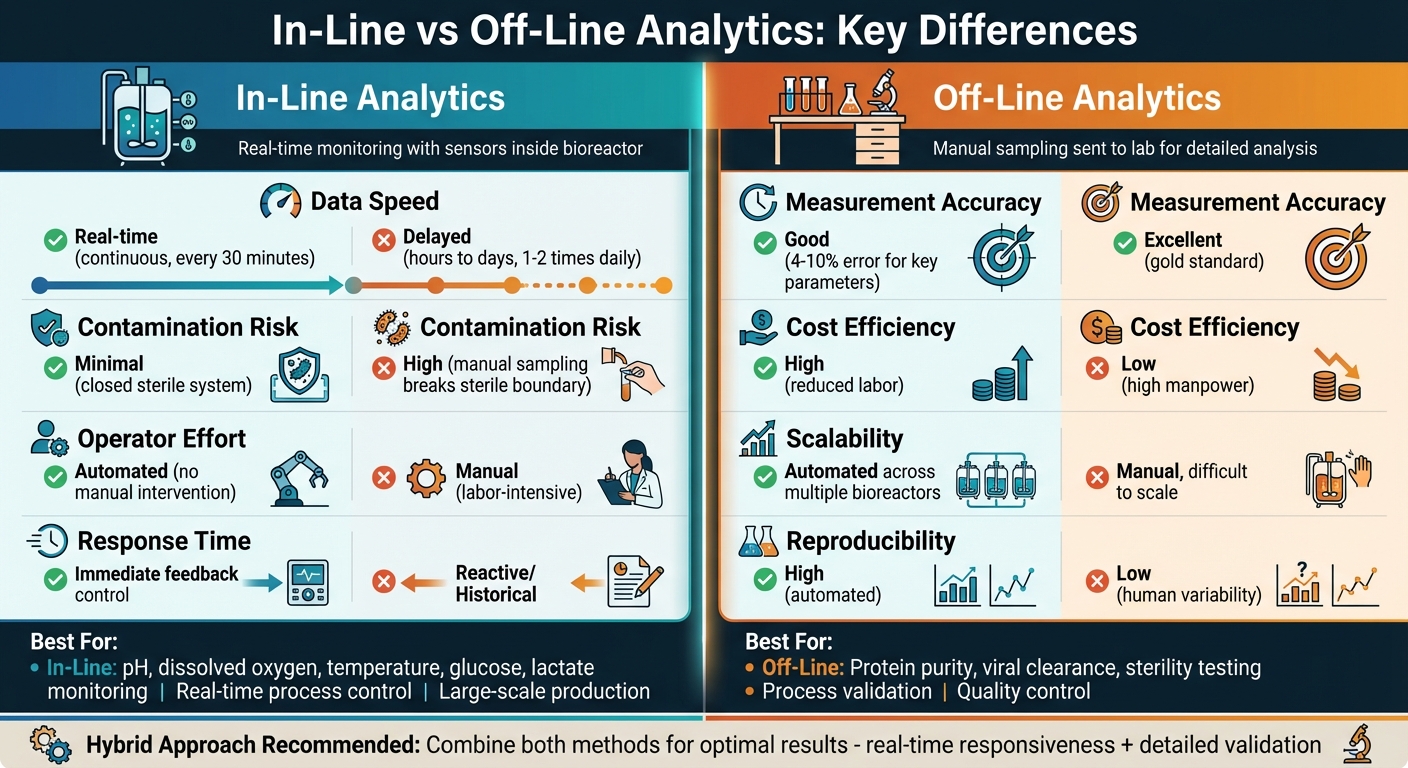
In-line analytics and off-line analytics are two methods used to monitor and control processes in cultivated meat production. The choice between them depends on your need for real-time data versus high-precision analysis. Here’s a quick breakdown:
- In-line analytics: Real-time monitoring with sensors inside the bioreactor. Provides instant data on factors like pH, dissolved oxygen, and glucose levels. Helps maintain sterile conditions and enables automated adjustments.
- Off-line analytics: Manual sampling sent to a lab for detailed analysis. Offers highly accurate results for complex parameters like purity and sterility but involves delays and higher contamination risks.
Key Differences:
- Speed: In-line provides instant feedback; off-line takes hours or days.
- Contamination: In-line minimises risk; off-line increases it due to manual handling.
- Labour: In-line is automated; off-line requires manual effort.
- Accuracy: In-line is precise but limited; off-line is the gold standard for complex tests.
Quick Comparison
| Factor | In-Line Analytics | Off-Line Analytics |
|---|---|---|
| Data Speed | Real-time | Delayed (hours to days) |
| Contamination Risk | Minimal | High |
| Labour Effort | Automated | Manual |
| Accuracy | Good for basic metrics | Excellent for complex tests |
A hybrid approach combining both methods can offer the best results, balancing real-time responsiveness with detailed validation.
In-Line vs Off-Line Analytics Comparison for Cultivated Meat Production
Bioprocess analytics and control
In-Line Analytics: How They Work
In cultivated meat production, keeping sterility intact and acting quickly to correct issues are absolutely critical. This is where in-line analytics come into play. These systems use sensors embedded directly in the bioreactor or process stream to continuously monitor the culture media. The beauty of this setup? It keeps sterility intact while providing instant data for automated control systems, ensuring smooth operation without interruptions [2].
Here’s how it works: sensors gather real-time data, and if key parameters - like glucose levels - drop below a threshold (e.g., 4 g/L), automated systems step in to make adjustments immediately [3]. Melissa Semple, a Senior Product Manager at Cytiva, explains that these in-line readings allow for rapid process control through automated closed-loop controllers [3].
The technology behind this includes electrochemical probes, capacitance sensors, and spectroscopic methods like Raman spectroscopy. These tools measure everything from environmental conditions to metabolic and cellular parameters with impressive accuracy. For instance, a 2024 study using the ProCellics™ Raman Analyser reported a 4% error margin for glucose monitoring, which enabled automated nutrient feeding and eliminated the need for manual sampling [4].
Durability is another key feature of these sensors. They are designed to endure harsh sterilisation methods, such as steam-in-place (SIP) or gamma irradiation, without losing calibration [3]. This resilience ensures uninterrupted production, making sensor selection a technical decision that depends on the type of bioreactor and sterilisation method being used.
Parameters Monitored with In-Line Analytics
In-line systems can track a broad range of parameters, from basic environmental metrics to complex biological indicators. Environmental sensors handle essentials like pH, dissolved oxygen (DO), temperature, and pressure - metrics that are fundamental to any cultivated meat process. Metabolic sensors focus on nutrients (e.g., glucose and glutamine) and waste products (e.g., lactate and ammonium), while cellular sensors, such as capacitance probes, measure viable and total cell density to monitor biomass and cell health in real time [3].
For advanced precision, spectroscopic tools deliver error margins between 4–10% for key metrics [4]. Take Raman spectroscopy, for example - it can predict total cell density with a 5% error and viable cell density with a 10% error. It also achieves 4% error for glucose, 8% for lactate, and 7% for ammonium. This level of accuracy allows producers to go beyond basic monitoring, enabling them to assess cellular functions and even product quality attributes like protein titre, integrity, and glycosylation patterns.
| Parameter Type | Specific Parameters | Common In-Line Technology |
|---|---|---|
| Environmental | pH, Dissolved Oxygen (DO), Temperature, Pressure | Electrochemical probes, Optical sensors |
| Metabolic Proxies | Glucose, Lactate, Glutamine, Ammonium | Raman Spectroscopy, NIR, Enzymatic probes |
| Cellular Attributes | Viable Cell Density (VCD), Total Cell Density (TCD) | Capacitance (Dielectric Spectroscopy), Raman |
| Product Quality | Titre, Protein integrity, Glycosylation | Raman Spectroscopy, MWIR Spectroscopy |
These systems do more than just measure - they provide operational benefits that improve efficiency and reliability.
Benefits of Real-Time Monitoring
The standout advantage of in-line analytics is the delivery of actionable data. Continuous measurement means operators can intervene before minor issues turn into major problems. This is especially crucial in long-duration, large-volume bioreactors, where early intervention can prevent significant product loss [2].
Real-time monitoring also makes scaling up production much easier. Larger volumes bring more complexity, but in-line sensors ensure precise control. Automated systems maintain stable glucose levels, avoid toxic metabolite build-up, and ensure consistent results across batches - all without requiring constant human oversight [4].
Another major benefit is reduced labour demands. Traditional manual sampling is time-consuming and requires skilled personnel. In contrast, automated in-line systems free up staff to focus on more strategic tasks, streamlining operations and boosting overall productivity [4].
Off-Line Analytics: How They Work
Off-line analytics rely on manual sampling to monitor cultivated meat production. This process involves an operator extracting a sample from the bioreactor or process stream and sending it to a Quality Control laboratory for analysis. In the lab, validated assays like High-Performance Liquid Chromatography (HPLC), purity tests, and sterility checks are performed under controlled conditions. The results are then logged into a Laboratory Information Management System (LIMS) for record-keeping and further use [1][5].
One major drawback of off-line methods is the delay in receiving results. Depending on the complexity of the tests, it can take hours, days, or even weeks to get the data back [1]. In traditional cultivated meat production, off-line sampling typically happens only once or twice per day [5]. By the time results are available, they reflect past conditions rather than offering immediate insights for process adjustments.
Despite these delays, off-line methods play a critical role in cultivated meat bioprocessing. They provide highly accurate data that is essential for calibrating and validating in-line sensors. These methods also help detect process deviations that automated probes might overlook. As John Carvell, Sales and Marketing Director at Aber Instruments, notes:
In some cases where the biomass method is already validated using an off-line method, the online probe can be used to pick up any process deviations or errors in the sample collection and analysis. [5]
The inherent lag in off-line analytics opens up a broader discussion about their accuracy and role in process validation.
Accuracy and Validation in Off-Line Methods
Off-line analytics shine when precision is non-negotiable. They serve as the gold standard for calibrating in-line sensors, ensuring that real-time measurements are reliable and that in-line data accurately reflects actual process conditions. These methods are particularly adept at assessing complex parameters like viral clearance, detailed purity profiles, and sterility testing - areas where in-line systems still fall short. As AMF has stated:
Off-line analysis provides precise insights into process parameters... this method is essential for complex applications requiring high accuracy, such as bioprocesses. [1]
This level of accuracy is especially crucial during process development and scale-up phases. For instance, in a study involving Raman spectroscopy, off-line measurements were used as the benchmark to correlate real-time in-line data with high-precision results [4]. This hybrid approach allows producers to evaluate Critical Process Parameters (CPPs) and address deviations before they escalate into larger issues.
However, achieving this level of precision comes with its own set of challenges.
Limitations of Discrete Sampling
While off-line analytics deliver exceptional accuracy, they also introduce several operational hurdles. One of the biggest risks is microbial contamination. Since manual sampling involves breaking the sterile boundary of the bioreactor, each sample collection increases the likelihood of contamination [2]. This risk can lead to costly batch failures, as Sigma-Aldrich highlights:
The requirement for frequent, manual sampling increases the risk of batch failures due to contamination. [4]
Another challenge is the labour-intensive nature of off-line sampling. From extracting the sample to conducting lab analysis, the process requires significant manual effort [5]. As a result, sampling frequency is typically limited to once or twice a day, leaving long gaps where process conditions remain unmonitored.
Additionally, off-line cell counts are prone to human variability, which reduces reproducibility compared to automated in-line systems. The time lag in off-line analysis also means that any detected deviations are identified too late, often after they have already caused significant issues [1].
| Factor | Off-Line Analytics | In-Line Analytics |
|---|---|---|
| Data Speed | Slow (Hours to Days) | Instant / Real-time |
| Contamination Risk | High (Manual sampling) | Zero (Inside sterile boundary) |
| Operator Effort | Very High | None |
| Actionability | Historical / Reactive | Immediate feedback |
| Reproducibility | Low (Human variability) | High |
Despite these limitations, off-line analytics remain an essential tool for validation and quality control in cultivated meat production. The key lies in knowing when to rely on off-line methods, balancing their precision with the need for real-time monitoring and process control.
In-Line vs Off-Line Analytics: Direct Comparison
When deciding between in-line and off-line analytics for cultivated meat production, it's crucial to understand how these methods differ. Each approach has its own strengths and weaknesses, influencing factors like process control, contamination risk, and operational efficiency.
A key difference lies in measurement frequency. In-line sensors deliver continuous, real-time data, while off-line methods depend on manual sampling, typically conducted just once or twice a day [4]. This disparity in data availability has a significant impact on how quickly producers can react to potential issues. As highlighted in Holloid's bioprocess monitoring guide:
A delay of a few hours in detecting a pH drift or a nutrient crash can mean the difference between a successful batch and millions of dollars in lost product. [2]
This real-time advantage of in-line analytics plays a pivotal role in ensuring timely interventions.
Contamination risk is another major point of contrast. Off-line sampling introduces a higher risk of contamination due to manual handling, whereas in-line sensors maintain a sterile environment by keeping the sample contained within the bioreactor [2].
From a cost perspective, operational efficiency and scalability also differ. In-line systems reduce labour demands and allow automated control across multiple bioreactors, making them more cost-effective [1][3]. In contrast, off-line methods struggle to scale efficiently due to reliance on manual sampling and increased operational effort [2].
Comparison Table: In-Line vs Off-Line Analytics
| Factor | In-Line Analytics | Off-Line Analytics |
|---|---|---|
| Measurement Frequency | Continuous (every 30 minutes) [4] | Low/Periodic (1–2 times daily) [4] |
| Data Availability | Instant, real-time [2] | Delayed (hours to weeks) [2] |
| Contamination Risk | Minimal (closed system) [2] | High (manual sampling) [2] |
| Response Time | Immediate feedback control [2] | Reactive, historical [2] |
| Operator Effort | Automated [1] | Manual [2] |
| Cost Efficiency | High (reduced labour) [1] | Low (high manpower) [1] |
| Scalability | Automated [3] | Manual [2] |
| Reproducibility | Automated [1] | Manual [2] |
| Measurement Accuracy | Good (4–10% error for key parameters) [4] | Excellent (gold standard) [1] |
The trend in the industry is clear: a shift from the reactive "Quality by Testing" model to the more proactive "Quality by Design" approach. This evolution underscores the preference for in-line solutions, which provide greater control and efficiency in cultivated meat production processes.
sbb-itb-ffee270
Applications in Cultivated Meat Bioprocessing
In cultivated meat production, both in-line and off-line methods play essential roles, each tailored to specific tasks.
In-Line Analytics
In-line sensors are vital for maintaining the core conditions needed for cell survival and growth. For instance, pH and dissolved oxygen probes provide continuous feedback, enabling automatic adjustments to aeration and agitation systems. Advanced tools like Raman spectroscopy take this a step further by monitoring key metrics - such as glucose, lactate, and ammonium - in real time. This allows automated feeds to kick in, preventing critical failures and ensuring smooth operations [4].
Off-Line Analytics
Off-line methods, on the other hand, handle more intricate quality assurance tasks that go beyond the capabilities of in-line systems. Tests for sterility, purity (using HPLC), and viral safety require laboratory analysis. During process development, off-line sampling is particularly valuable for building predictive models that enhance the accuracy of in-line sensors.
Hybrid Approach
By combining the strengths of both methods, a hybrid approach offers the best of both worlds: the immediacy of in-line monitoring and the precision of off-line validation. This synergy enables more effective process control, ensuring both real-time responsiveness and high-fidelity accuracy [2].
When to Use In-Line Analytics
In-line sensors become indispensable when real-time data is critical to the success of a batch. For example, in large-scale bioreactors, continuous monitoring of pH and dissolved oxygen ensures optimal conditions for cell growth. Even brief delays in detecting deviations could lead to losses worth millions of pounds [2].
Real-time data also supports closed-loop feeding systems. Raman spectroscopy, for instance, predicts glucose levels with a 4% error margin, lactate with 8%, and ammonium with 7% [4]. This level of precision helps maintain steady-state conditions without manual intervention, boosting both yield and consistency.
Technologies like capacitance or Doppler ultrasound enable continuous monitoring of viable cell density, ensuring that cells are harvested at the right time. The industry’s shift towards Quality by Design is further supported by in-line analytics. As Sigma-Aldrich explains:
Implementing process analytical technology (PAT) for automated in-line, real-time measurements allows to navigate cell culture processes with an improved process understanding and decreased process risk, enabling more advanced process control. [4]
When to Use Off-Line Analytics
Off-line methods are the go-to choice when accuracy is more important than immediacy. For example, final product validation relies on the laboratory-grade precision that in-line sensors currently cannot achieve [2].
In the early stages of process development, frequent off-line sampling helps correlate in-line sensor readings with laboratory gold standards. This builds the predictive models needed for automated control. Off-line methods also act as a quality control checkpoint, ensuring that issues like sensor drift or fouling don’t compromise the reliability of in-line data [6].
Choosing between in-line and off-line methods requires a careful balance between the need for real-time data and the demand for precise accuracy. Each approach has its strengths, and their combined use often delivers the best results.
Choosing Between In-Line and Off-Line Analytics
Factors to Consider When Selecting Analytics Methods
Deciding between in-line and off-line analytics comes down to a few key considerations. In-line measurements provide real-time data in milliseconds, making them ideal for automated closed-loop control systems. On the other hand, off-line methods - which can take hours or even days - offer highly precise analytics but lack the immediacy needed for on-the-spot process adjustments. This delay makes off-line data more suited for historical analysis rather than real-time decision-making [7].
Another critical factor is contamination risk. In-line sensors stay within the sterile environment of the bioreactor, preserving its integrity. In contrast, off-line methods involve manual sampling, which introduces the potential for contamination. As Sigma-Aldrich highlights:
The requirement for frequent, manual sampling increases the risk of batch failures due to contamination [4].
The ability to detect and address errors in real time is another advantage of in-line analytics. As Christopher Kistler, Fellow Scientist at Catalent Biologics, points out:
Processing errors can be detected as they happen, and mitigated before they have the opportunity to become catastrophic [3].
Parameter complexity also plays a role. Basic parameters like pH, dissolved oxygen, and temperature are typically monitored in-line. However, more intricate measurements - such as protein purity, viral clearance, or specific amino acid profiles - often require advanced off-line assays [3]. Lastly, the durability of sensors under bioreactor conditions is a practical concern. If an in-line sensor fails mid-process, replacing it without compromising the sterile boundary is nearly impossible [7] [3]. This makes reliability a crucial factor to weigh [2].
These factors are essential when choosing the right analytics approach for cultivated meat production.
How Cellbase Supports Analytics Equipment Procurement
Cellbase helps teams navigate these challenges by connecting them with verified suppliers for both in-line and off-line analytics equipment. Whether you need in-line sensors like pH probes, dissolved oxygen monitors, or Raman spectroscopy systems, or off-line instruments designed for cultivated meat production, Cellbase simplifies the process.
Each listing includes detailed use-case specifications, making it easy to find equipment that works with your bioreactor - whether it’s a stirred-tank, airlift, or single-use system. Transparent pricing and direct communication with suppliers streamline procurement. For teams moving from off-line to in-line monitoring, Cellbase also provides a marketplace for SIP/CIP-compatible sensors and PAT solutions, reducing the complexity of upgrading your analytics setup.
Conclusion
In-line and off-line analytics each bring distinct advantages to cultivated meat production. In-line sensors provide real-time data without compromising sterility, allowing automated control over critical factors like pH, dissolved oxygen, and temperature. As Holloid points out, even a few hours' delay in identifying issues like pH drift or nutrient depletion can result in losses worth millions [2]. These sensors must also endure sterilisation cycles, as mid-production replacements are not feasible.
On the other hand, off-line analytics are unmatched when it comes to precision. Advanced assays, such as those for protein purity or viral clearance, cannot be performed on-site. While these methods deliver highly accurate results, they often take hours or even days to complete. Additionally, manual sampling poses risks of contamination and variability due to human error.
A hybrid approach, combining real-time in-line monitoring with precise off-line validation, allows for a transition from Quality by Testing to Quality by Design. This integrated strategy is further supported by tailored procurement solutions.
Given these analytical contrasts, choosing the right equipment becomes essential. Cellbase streamlines this process by connecting cultivated meat producers with trusted suppliers. Whether your needs include SIP/CIP-compatible sensors for real-time monitoring or advanced LC-MS systems for complex assays, Cellbase provides transparent pricing and detailed, bioprocess-specific specifications. By selecting the appropriate tools, producers can achieve greater process consistency and ensure the quality of cultivated meat products.
FAQs
What are the benefits of combining in-line and off-line analytics in cultivated meat production?
Using a mix of in-line and off-line analytics brings clear benefits to cultivated meat bioprocessing. In-line analytics deliver real-time data straight from the bioreactor, enabling instant tracking and control of crucial parameters like pH, dissolved oxygen, and cell viability. This ensures the process stays stable and helps maintain a consistent level of product quality.
On the other hand, off-line analytics involve lab-based testing of samples, providing deeper insights into factors such as cell health, metabolite levels, and potential contamination - things that can’t always be measured in real time. By combining these two approaches, producers can enjoy the real-time benefits of in-line monitoring while using the detailed insights from off-line analysis for quality control and problem-solving.
This dual strategy improves process reliability, minimises contamination risks, and ensures compliance with regulatory standards. It becomes particularly crucial during scale-up and commercial production, where efficiency and quality must go hand in hand. Tools like Cellbase can assist professionals in the field by offering the resources needed to implement this approach successfully.
What role do in-line analytics play in ensuring sterility during cultivated meat production?
In-line analytics are essential for maintaining sterility during cultivated meat production. They enable continuous, real-time monitoring directly within the bioreactor or process stream, removing the need for manual sampling - a step that could introduce contamination. This ensures that the production environment remains tightly controlled at all times.
With the use of in-line sensors, key data points like pH, temperature, and nutrient levels can be monitored without breaking the sterile barrier. This technology is a key factor in maintaining both product consistency and safety throughout the cultivated meat production process.
Why is off-line analytics preferred for complex testing in cultivated meat production?
Off-line analytics plays a key role in cultivated meat production, especially when it comes to complex testing. This approach relies on laboratory-based techniques, which are designed to provide precise and detailed results. By focusing on critical parameters, it ensures thorough quality control and reliable validation processes.
Although in-line methods are better suited for real-time monitoring due to their speed, off-line analytics stands out when precision and comprehensive data are priorities. Its ability to handle intricate tests makes it indispensable for upholding the rigorous standards demanded in cultivated meat production.











