Monitoring live cells in bioreactors is critical for cultivated meat production. Scaling requires precise tools to track cell health and growth in real time. This article reviews key methods, including capacitance sensors, Raman spectroscopy, and fluorescence, highlighting their strengths and limitations for industrial applications.
Key Insights:
- Capacitance Sensors: Measure viable cell density continuously. Effective for adherent cells but sensitive to cell size changes.
- Raman Spectroscopy: Tracks metabolites like glucose and lactate. Ideal for aqueous environments but requires complex calibration.
- Fluorescence: Monitors metabolic activity via NADH/NADPH signals. Fast but affected by media background signals.
Challenges:
- Traditional tests like Trypan Blue are destructive and slow.
- High cell densities and complex media interfere with optical methods.
- Sensor fouling and calibration needs limit efficiency.
Choosing the right method depends on process requirements, bioreactor scale, and sterility needs. For large-scale operations, combining multiple techniques often yields the best results.
Capacitance-Based Sensors for Viable Cell Density
How Dielectric Spectroscopy Works
Capacitance sensors, also known as radio-frequency impedance sensors, treat living cells as if they were tiny spherical capacitors. When an electric field is applied to a suspension of cells, ions in the culture medium and within the cell cytoplasm start to move. They eventually encounter the non-conductive plasma membrane, causing polarisation - a separation of charges across the membrane [5][6].
Here’s the key: only cells with intact membranes can polarise. Dead cells, which lack intact membranes, cannot trap ions and, therefore, don’t contribute to the capacitance signal [5][7]. John Carvell, Sales and Marketing Director at Aber Instruments Ltd., explains this well:
"Radio-frequency (RF) impedance... is generally regarded as the most robust and reliable method to monitor live-cell concentrations in mammalian cell culture." [5]
Dielectric spectroscopy builds on this by measuring the dielectric properties (or permittivity) of the cell suspension across various frequencies. This process generates a β-dispersion curve, illustrating how the cells’ ability to polarise decreases as the electric field frequency rises [6]. A single-frequency reading often reflects the viable biovolume - the total volume occupied by living cells - rather than just the number of cells. Larger cells naturally contribute more to the signal than smaller ones [5][6].
These principles form the backbone of capacitance sensor technology, making it a valuable tool in bioreactor systems.
Using Capacitance Sensors in Cultivated Meat Bioreactors
Capacitance sensors are compatible with both single-use and multi-use bioreactor systems. For single-use setups, disposable sensor discs can be welded into flexible film bags or inserted through pre-fitted tube ports [5][9]. In stainless steel systems, reusable 12-mm probes are connected via sterile ports [9].
A practical example comes from Aachen University, where researchers used the BioPAT ViaMass system in a 20-litre rocking-motion single-use bioreactor to monitor CHO DG44 cells. They achieved a strong correlation (regression coefficient of 0.95) between capacitance readings and total cell volume [5]. Similarly, Xpand Biotechnology in The Netherlands employed Aber biomass sensors in their Scinus cell expansion system to track mesenchymal stem cells (MSCs) grown on microcarriers at a density of 60 g/L. The sensors effectively traced growth profiles across volumes ranging from 150 mL to 1 litre, with results aligning closely with offline reference measurements [5].
For cultivated meat production, capacitance sensors shine when working with adherent cells on microcarriers. Unlike optical methods, which can struggle with solid carriers, capacitance sensors can penetrate these structures. This capability makes them particularly useful for monitoring anchorage-dependent cells, a cornerstone of cultivated meat manufacturing [8].
Strengths and Weaknesses of Capacitance Sensors
Capacitance sensors offer continuous, real-time data without the contamination risks or delays associated with manual sampling. They are currently the only commercially available online tools for assessing cell viability in industrial bioprocesses [7]. While traditional offline methods like trypan blue assays have a relative error of around 10%, capacitance frequency scanning can reduce this error to between 5.5% and 11% [6].
That said, these sensors do have their limitations. Single-frequency measurements can’t differentiate between an increase in cell number and an increase in cell size. For instance, if cells grow significantly in diameter during a run - whether due to stress or the death phase - the signal might misrepresent the actual cell count unless multi-frequency scanning is used [6]. Additionally, changes in the suspension medium, such as feed additions or dilutions, can cause temporary "dips" in the data that don’t reflect real biomass changes [5]. In rocking-motion bioreactors, the sensor can momentarily encounter the gaseous headspace, requiring advanced filter algorithms to avoid signal interference [5].
These factors are crucial when fine-tuning live-cell monitoring for cultivated meat production.
Spectroscopy Methods for Live-Cell Analysis
Raman and NIR Spectroscopy
Raman spectroscopy uses inelastic light scattering from a 785 nm laser to generate a molecular fingerprint, allowing simultaneous measurement of metabolites like glucose, lactate, glutamine, and ammonium. On the other hand, NIR spectroscopy (800–2,500 nm) detects optical absorptions from overtones and combination bands [10][12][13][14]. Raman's minimal sensitivity to water makes it ideal for aqueous environments like cell cultures, whereas NIR's high water sensitivity - due to the strong O–H stretch signal - can obscure critical biochemical data [10][12][14].
In March 2017, Lonza Biologics compared NIR, Raman, and 2D-fluorescence in 15 mL miniature bioreactors (ambr™ system). They found Raman to be the most reliable for measuring lactate and glucose, while NIR performed better for predicting glutamine and ammonium ion levels [10][11].
In April 2022, researchers at Sartorius Stedim Biotech integrated an in-line Raman flow cell into the cell-free harvest stream of a CHO cell perfusion process. Using a HyperFluxPRO Raman spectrometer with a 785 nm laser, they achieved automated glucose feedback control, maintaining concentrations at 4 g/L and 1.5 g/L with a variability of ±0.4 g/L over several days [13]. J. Lemke from Sartorius Stedim Biotech noted:
"The results demonstrate the high potential of Raman spectroscopy for advanced process monitoring and control of a perfusion process with a bioreactor and scale-independent measurement method." [13]
In May 2011, Bristol-Myers Squibb used an in-line Raman probe in 500-litre bioreactors to monitor multiple parameters, including glutamine, glutamate, glucose, lactate, ammonium, viable cell density (VCD), and total cell density (TCD). Spectra were collected every two hours with a Kaiser Optical Systems RamanRXN3 instrument, showcasing Raman's ability to track nutrient increases and metabolite decreases during feed additions in large-scale manufacturing [14].
While Raman and NIR spectroscopy deliver detailed chemical insights, fluorescence and UV-Vis methods offer complementary perspectives on cellular metabolism and biomass.
Fluorescence and UV-Vis Spectroscopy
UV-Vis spectroscopy measures light absorbance or scattering to estimate total biomass [16]. This straightforward and widely-used method, however, struggles to differentiate between viable and dead cells and becomes less accurate at higher cell densities [16].
Fluorometry, which is more sensitive than UV-Vis, focuses on specific intracellular markers like NADH and NADPH, indicators of metabolic activity. In situ fluorometry uses 366 nm ultraviolet light to excite NADH/NADPH, which then fluoresces at around 460 nm [16]. Veer Pramod Perwez explains:
"The only continuous monitoring strategy so far developed that provides information on the biochemical or metabolic state of the cell population is in situ fluorometry." [16]
In cultivated meat production, where real-time data is essential, fluorescence delivers rapid feedback on metabolic changes, while UV-Vis offers an economical way to estimate biomass. Fluorescence can track metabolic shifts and detect substrate depletion in real time by monitoring NADH levels. For instance, in one study, 2D-fluorescence measured ammonium concentrations with an RMSECV of 0.031 g/L, outperforming both Raman and NIR in miniature bioreactor setups [11]. Additionally, automated microfluidic platforms can combine brightfield microscopy (to measure total cell concentration) with fluorescence detection using propidium iodide, determining cell viability in just 10.3 minutes [15].
Comparing Different Spectroscopy Methods
When comparing these techniques, each has distinct strengths for bioreactor monitoring. Raman stands out for its ability to predict glucose, lactate, and antibody titres, thanks to its molecular fingerprinting and low interference from water [10][11]. NIR, despite its sensitivity to water, is more effective for monitoring glutamine and ammonium [10][12]. Fluorescence provides detailed insights into metabolic activity and viability, while UV-Vis remains a simple and cost-efficient choice for estimating total biomass [16].
Multivariate analysis enhances the interpretation of complex spectra, enabling the simultaneous monitoring of multiple analytes [10][13][14]. For cultivated meat production, selecting the right spectroscopy method depends on the metabolites to monitor, the bioreactor's scale, and whether single-use or multi-use systems are employed. These techniques collectively enable precise cell monitoring, with Raman's compatibility with aqueous environments and its multi-analyte capabilities making it particularly appealing for large-scale operations [13][14].
Mammalian Cell Culture - Raman as a Means of Monitoring & Controlling Upstream Bioprocesses
sbb-itb-ffee270
Advanced Methods for Cell Physiology and Viability
In addition to spectroscopy, cutting-edge techniques offer deeper insights into cell physiology and viability.
FTIR for Cell Viability and Apoptosis Monitoring
FTIR spectroscopy uses molecular vibrations in proteins, lipids, and carbohydrates to detect nutrient stress and early apoptosis, both critical markers of declining cell health in cultivated meat bioreactors.
One approach, ATR-FTIR (Attenuated Total Reflection), analyses spectral variability in high-wavenumber regions to differentiate between healthy and nutrient-deficient cells. In May 2024, researchers at Dxcover Ltd. employed an ATR-FTIR platform fitted with disposable internal reflection elements (IREs) to monitor CHO cell health. Using Principal Component Analysis (PCA), they successfully distinguished healthy cells from nutrient-deficient ones in PC space. The platform achieved impressive multi-output R² values close to 0.98 for glucose and lactic acid, offering real-time insights into cell viability [17]. Since lactic acid build-up can lead to cell death, this real-time monitoring allows for timely interventions to sustain cell health.
Modern FTIR systems are designed with disposable IREs or submerged probes for direct integration into bioreactor environments. This setup not only provides real-time data but also reduces contamination risks [17]. As highlighted in Frontiers in Bioengineering and Biotechnology:
"Spectroscopy-based technologies are well suited as PAT approaches as they are non-destructive and require minimum sample preparation." [17]
Expanding on these capabilities, multi-frequency capacitance scanning addresses the limitations of single-frequency methods.
Multi-Frequency Capacitance Scanning
While single-frequency capacitance sensors are useful for measuring viable cell volume (VCV), they struggle to distinguish between changes in cell size and cell count. This limitation becomes especially problematic during apoptosis, when cell diameters often increase [18]. Multi-frequency capacitance scanning resolves this issue by measuring permittivity across a range of 50–20,000 kHz, capturing the β-dispersion curve to accurately assess viable cell concentrations regardless of size variations [18].
In October 2019, researchers at Sartorius Stedim Biotech utilised an Aber Instruments FUTURA pico probe to monitor DG44 CHO cells in 250 mL bioreactors. By applying Orthogonal Partial Least Squares (OPLS) modelling to 25 discrete frequencies, they reduced VCC prediction errors to just 5.5% to 11%, a significant improvement over the 16% to 23% error rates seen with single-frequency measurements [18]. The model effectively tracked cell concentrations exceeding 10 million cells/mL and quickly identified deviations caused by dilution and feeding changes, with error margins of 6.7% to 13.2% [18].
The characteristic frequency (fC), which indicates the point where cellular polarisation is half complete, shifts based on cell size and polarisability. This provides an additional marker for physiological changes, particularly during the cell death phase when morphology undergoes notable transformations [18]. As Analytical and Bioanalytical Chemistry explains:
"The influences of VCC and cell diameter on the permittivity signal are not distinguishable with one frequency measurement." [18]
Comparing Analytical Methods for Live-Cell Monitoring
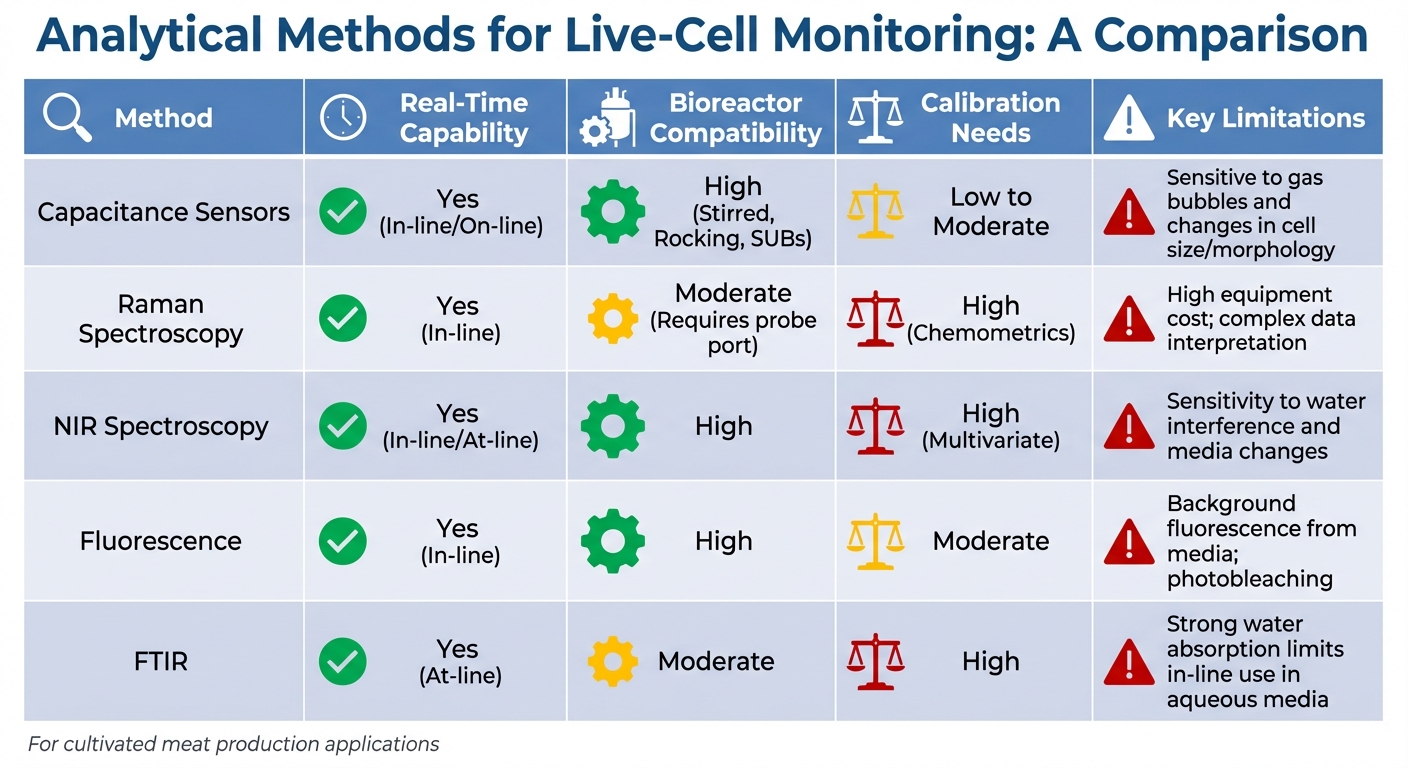
Comparison of Analytical Methods for Live-Cell Monitoring in Bioreactors
This section takes a closer look at key analytical methods used for live-cell monitoring in cultivated meat bioreactors, building on the advanced techniques previously discussed.
Selecting the best method involves balancing accuracy, speed, and practicality. Each technique offers distinct strengths, whether it’s tracking viable cell density, monitoring metabolic activity, or maintaining sterility in single-use systems.
Capacitance-based sensors are currently the only commercially available on-line option tailored for viability monitoring [7]. These sensors measure viable cell volume by detecting the polarisation of cells with intact membranes in an alternating electric field. While single-frequency systems may struggle with accuracy when cell sizes vary, multi-frequency scanning significantly improves precision, achieving error margins of 5.5%–11% [18].
Spectroscopic methods - such as Raman, NIR, and fluorescence spectroscopy - offer a more comprehensive view of metabolic activity, tracking multiple parameters alongside biomass. These methods are non-invasive, making them ideal for single-use bioreactors where sterility is critical. However, they come with challenges: spectroscopic systems require extensive calibration with chemometric models and often involve higher initial costs compared to capacitance probes.
FTIR spectroscopy is particularly effective at detecting early signs of apoptosis and nutrient stress through molecular vibration analysis. However, its strong water absorption limits its utility for continuous in-line monitoring in aqueous environments [7]. Instead, FTIR works best as an at-line method, especially when paired with multivariate analysis for real-time metabolite tracking.
Analytical Methods Comparison Table
| Method | Real-Time Capability | Bioreactor Compatibility | Calibration Needs | Key Limitations |
|---|---|---|---|---|
| Capacitance Sensors | Yes (In-line/On-line) | High (Stirred, Rocking, SUBs) | Low to Moderate | Sensitive to gas bubbles and changes in cell size/morphology |
| Raman Spectroscopy | Yes (In-line) | Moderate (Requires probe port) | High (Chemometrics) | High equipment cost; complex data interpretation |
| NIR Spectroscopy | Yes (In-line/At-line) | High | High (Multivariate) | Sensitivity to water interference and media changes |
| Fluorescence | Yes (In-line) | High | Moderate | Background fluorescence from media; photobleaching |
| FTIR | Yes (At-line) | Moderate | High | Strong water absorption limits in-line use in aqueous media |
For cultivated meat production, where precision and reliability are non-negotiable, matching analytical methods to specific process requirements is key to achieving optimal bioreactor performance. Platforms like Cellbase can simplify the decision-making process by streamlining equipment selection.
Conclusion and Recommendations
Selecting the right analytical method involves balancing process requirements with factors like scale, cost, and regulatory demands. Your choice will depend on key considerations such as whether your cells are adherent or suspension-adapted, how often monitoring is needed, and how much invasiveness can be tolerated while ensuring sterility remains intact [1]. With the substantial cell demands of cultivated meat production [1], precision in monitoring is non-negotiable.
Key Factors for Choosing Analytical Methods
Real-time monitoring should be a top priority. Online systems allow for in situ data collection without removing samples, making them more efficient and less prone to errors compared to offline methods, which are labour-intensive and risk contamination [3][1]. For large-scale bioreactors - up to 2,000 litres or more - non-invasive techniques like Raman or NIR spectroscopy are especially useful. These methods are reagent-free and can track multiple parameters, such as glucose, lactate, and amino acids, simultaneously [1][3]. This multivariate capability not only reduces monitoring costs but also maintains the sterile, food-grade environment needed for regulatory compliance [19].
Sensitivity and dynamic range are equally important when analysing complex biological media. Luminescence-based assays generally offer higher sensitivity than fluorescence or absorbance methods [2]. Meanwhile, advanced spectroscopic techniques generate complex datasets that often require machine learning or chemometric tools for proper analysis [3][1]. For a simpler solution, capacitance-based sensors are effective for monitoring cell viability.
Scalability and regulatory compliance are essential for commercial production. Sensors in these settings must endure high-temperature sterilisation, minimise leaching, and operate for extended periods without needing recalibration. Automated, image-based tracking systems can also provide timestamped, audit-ready documentation, which is crucial for regulatory submissions to bodies like the FDA and EMA [4]. These requirements highlight the importance of sourcing the right equipment from specialised suppliers.
Streamlining Equipment Sourcing with Cellbase
Given the technical and regulatory complexities, finding the right analytical equipment is critical. General lab platforms often lack the expertise tailored to the cultivated meat industry. Cellbase addresses this gap as the first specialised B2B marketplace designed exclusively for cultivated meat production. It connects researchers, production managers, and procurement teams with verified suppliers offering bioreactors, sensors, growth media, and other essential tools. Each product listing is clearly tagged with specific use-case details - such as scaffold compatibility, serum-free formulations, or GMP compliance - making it easier to identify the right fit. By simplifying procurement and providing industry-specific insights, Cellbase helps reduce technical risks and accelerates decision-making, whether you're working on a small bench-scale project or scaling up to commercial production.
FAQs
What are the benefits of using capacitance sensors in bioreactors for cultivated meat production?
Capacitance sensors provide a real-time, non-intrusive way to measure viable cell biomass in bioreactors. They deliver precise and reliable data without interrupting the process, making them an excellent choice for tracking cell growth and health.
These sensors work seamlessly across systems of all sizes, from small-scale setups to large single-use industrial bioreactors. This flexibility improves process management, minimises reliance on offline sampling, and streamlines production workflows. By offering detailed insights into cell activity, capacitance sensors play a key role in refining bioprocesses, particularly for cultivated meat production.
What are the advantages of Raman spectroscopy for monitoring cell metabolites in bioreactors?
Raman spectroscopy allows for real-time, non-invasive tracking of crucial cell metabolites directly within bioreactors. This approach eliminates the need to withdraw samples, significantly reducing the risk of contamination. It can simultaneously measure a range of compounds, such as glucose, lactate, ammonium, and product titres, making it an efficient tool for extended processes like perfusion runs.
When compared to other methods, Raman spectroscopy often delivers higher precision for key metabolites like glucose and lactate. It can even outperform techniques such as near-infrared (NIR) and 2D fluorescence under certain conditions. Unlike traditional off-line methods, such as HPLC or colourimetric assays, Raman spectroscopy works continuously, cutting down on time and resource use while preserving the integrity of the cell culture.
In cultivated meat production, Raman spectroscopy stands out due to its compatibility with compact bioreactors and its ability to provide reliable, calibration-free measurements. For those in need of Raman-based monitoring tools, Cellbase offers a dependable marketplace for equipment tailored to cultivated meat production.
What are the challenges of using optical methods in bioreactors with high cell densities?
In environments with high cell density, optical methods face challenges like increased light scattering and media turbidity, which can skew measurements. Adding to the complexity, the build-up of cell debris can weaken signals and cause non-linear responses, making accurate readings even harder to achieve.
These issues are particularly problematic in bioreactors, where conditions are constantly changing and intricate. To address these limitations and maintain dependable monitoring, more sophisticated analytical techniques may be required.











