Process Analytical Technology (PAT) integrates real-time quality monitoring into manufacturing processes, improving consistency and reducing waste. It’s especially useful in cultivated meat production, where precise control of factors like pH, oxygen, and nutrients is critical. PAT combines in-line sensors, chemometrics, and automated systems to ensure product quality while meeting regulatory standards.
Key Steps to Implement PAT:
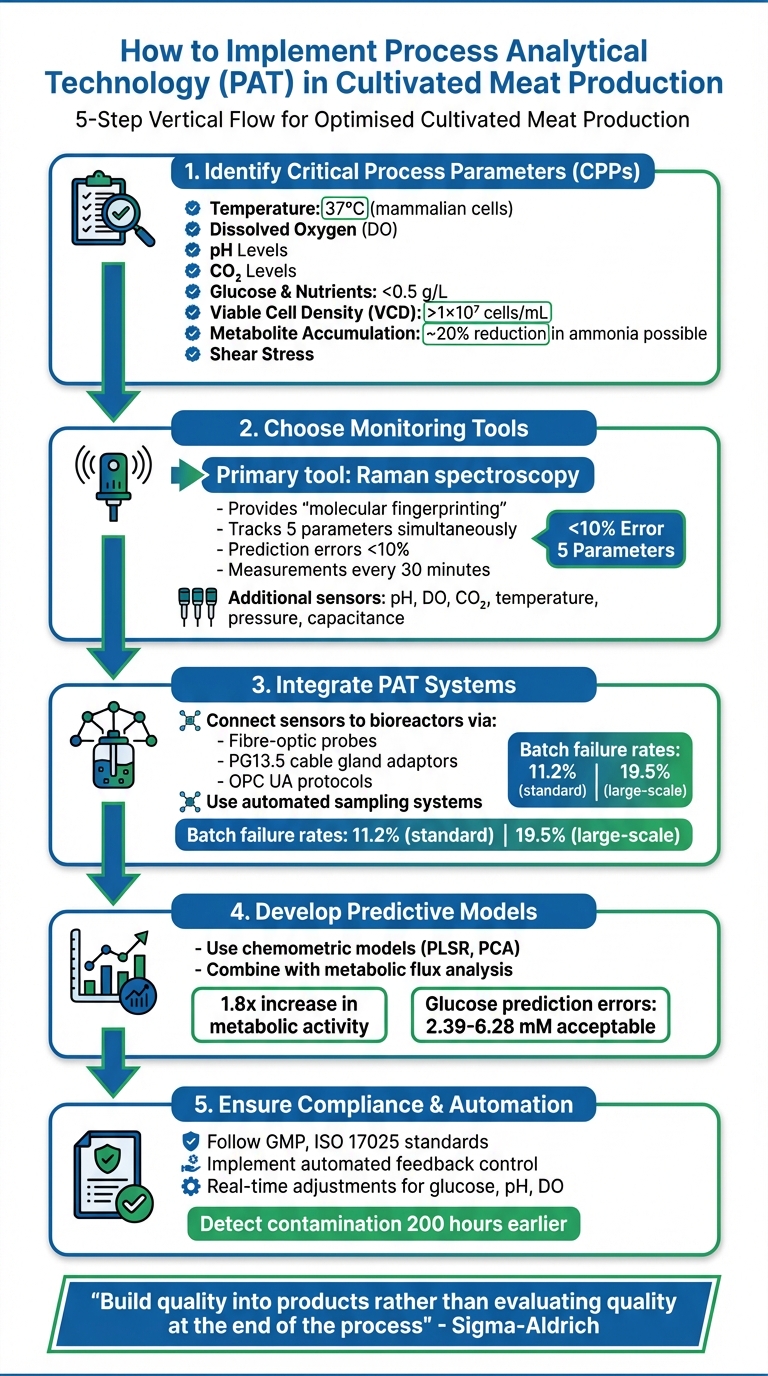
- Identify Critical Process Parameters (CPPs): Focus on factors like temperature, dissolved oxygen, pH, and glucose.
- Choose Monitoring Tools: Use in-line sensors (e.g., Raman spectroscopy) for real-time data.
- Integrate PAT Systems: Connect sensors to bioreactors for automated feedback control.
- Develop Predictive Models: Use data analysis to optimise processes.
- Ensure Compliance: Follow GMP, ISO 17025, and other regulatory guidelines.
Platforms like Cellbase simplify equipment sourcing for cultivated meat production, offering tools tailored to industry needs. By adopting PAT, you can improve efficiency, reduce costs, and maintain high product standards.
5-Step Process for Implementing PAT in Cultivated Meat Production
Bioprocessing Expert Panel Discussion I - PAT Implementation
Identifying Critical Process Parameters (CPPs)
To ensure success in cultivated meat production, it’s essential to identify the Critical Process Parameters (CPPs) that influence cell viability, biomass yield, and product quality. Mismanaging these can jeopardise entire production runs.
Key Parameters to Monitor
Temperature is a key factor. Mammalian cells thrive at around 37°C, while fish and insect cells require much cooler environments to maintain optimal metabolic activity [2].
Dissolved Oxygen (DO) is another critical element for aerobic metabolism. As production scales up, ensuring sufficient oxygen transfer becomes more challenging [2]. Without enough oxygen, cells may switch to anaerobic metabolism, leading to lactic acid buildup, which can hinder growth.
pH Levels are a window into the metabolic state of the culture. Any fluctuations can disrupt enzyme activity, harm cell health, and affect the product's characteristics, such as texture and water-holding capacity [2][3].
Carbon Dioxide (CO₂) levels must be carefully managed, especially in large-scale operations. Animal cells are particularly sensitive to elevated CO₂ levels, making constant monitoring essential [2].
Glucose and Nutrients are the main energy sources for cells. If glucose levels drop too low, cells may starve, leading to death or premature differentiation [2]. Keeping glucose concentrations low (e.g., below 0.5 g/L) can prevent inefficient metabolism and reduce lactate accumulation [4].
Viable Cell Density (VCD) helps track the culture's growth phases - lag, log, and stationary - allowing for the determination of the best harvest time [2]. For cultivated meat, high cell densities are often considered to be above 1×10⁷ cells/mL [2].
Metabolite Accumulation - such as ammonia and lactic acid - can hinder growth and reduce cell viability. Monitoring and controlling these toxic byproducts is crucial. For instance, one method achieved a 20% reduction in toxic ammonia levels [2].
Shear Stress caused by impellers or gas bubbles poses a unique challenge. Unlike microbial cells, animal cells lack a protective cell wall, making them more vulnerable to damage. Tolerable stress levels vary depending on the species and must be fine-tuned for each cell line [2].
These parameters provide the foundation for optimising cultivated meat production.
Parameters Specific to Cultivated Meat
While the above factors apply broadly, cultivated meat production introduces unique challenges that require special attention.
CO₂ Sensitivity is particularly important. Animal cells used in food production are more susceptible to CO₂ inhibition compared to microbial cells, making this a critical parameter to manage [2].
Scaling Up Production brings new priorities. In biopharma, bioreactors typically max out at 20,000 L for high-value products. However, cultivated meat will need significantly larger volumes to remain economically feasible. To put it into perspective, the largest microbial bioreactor ever built holds 1,500,000 L - a scale that cultivated meat production may one day need to reach [2].
Thermal Management varies by species. Non-mammalian cells require entirely different heating and cooling systems, making temperature control highly species-specific [2]. This variability demands flexible Process Analytical Technology (PAT) systems.
Finally, sourcing the right monitoring equipment for these parameters can be tricky. Platforms like Cellbase provide access to verified suppliers of sensors, probes, and analytical tools tailored for cultivated meat production.
Mastering these CPPs is a necessary step before implementing real-time control systems through PAT tools.
Selecting and Integrating PAT Tools
Once you've pinpointed the critical process parameters, the next step is selecting sensors that align with your needs - especially in terms of where measurements are taken and how quickly they respond. In-line monitoring stands out here. Since the sensors stay within the process stream, they provide the fastest and most dynamic real-time insights compared to at-line or off-line methods [6]. For parameters like pH or dissolved oxygen, which demand immediate feedback, in-line sensors eliminate delays caused by sampling.
Choosing Sensors and Technologies
One standout tool in this field is Raman spectroscopy, a go-to choice for cultivated meat production. Its ability to deliver "molecular fingerprinting" makes it particularly effective for identifying organic molecules like glucose and lactate, while being unaffected by water [6][7]. A study by Merck/Sigma-Aldrich in January 2026 highlighted the effectiveness of the ProCellics™ Raman Analyser and Bio4C® PAT Raman Software. This system monitored a CHO cell culture in a 3L water-jacketed bioreactor, taking measurements every 30 minutes. Notably, it tracked five parameters simultaneously and accurately distinguished between total and viable cell densities during a cell dilution event on day six, with a margin of error under 10% [11].
"Raman has become a first-choice PAT for monitoring and controlling upstream bioprocesses because it facilitates advanced process control and enables consistent process quality." - Karen A Esmonde-White, Endress+Hauser [8]
Raman spectroscopy isn't just precise; it predicts key metabolite levels with errors below 10% [7][11]. But Raman alone isn’t enough. You'll also need standard bioreactor sensors for pH, dissolved oxygen, CO₂, temperature, pressure, and capacitance [10][6]. To streamline operations and reduce contamination risks - especially since batch failure rates in cultivated meat production hover around 11.2%, rising to 19.5% in larger-scale setups - automated sampling systems are indispensable [5].
When selecting sensors, ensure they’re compatible with Multivariate Data Analysis (MVDA) and Design of Experiments (DOE) software [1]. This compatibility ensures tools can scale from small R&D bioreactors to full-scale commercial production [1].
Integrating PAT Tools into Bioreactor Systems
Modern bioreactor systems simplify the integration of PAT tools. Non-destructive, in-line measurements are made possible using fibre-optic probes, which are mounted via standard PG13.5 cable gland adaptors. These probes connect seamlessly to bioreactor systems through OPC UA protocols [8][9][11][1].
On the data side, platforms like Bio4C® PAT Raman Software or BioPAT® MFCS process sensor data into actionable insights for real-time control [10][11]. These systems use advanced tools like Principal Component Analysis (PCA) and Partial Least Squares (PLS) to convert complex spectral data into meaningful process parameters [9].
"The application of Raman technology... empowers comprehensive process understanding and control in biopharmaceutical manufacturing, enabling users to make the right decisions with confidence." - Merck/Sigma-Aldrich [11]
When building Raman models, techniques like analyte spiking - where known concentrations of analytes are added - help break correlations between compounds, ensuring the model doesn't rely on indirect trends [1]. Incorporating a broad range of process conditions using DOE ensures models are robust enough to handle commercial-scale variations [1].
With integration challenges addressed, the next task is sourcing the right PAT equipment.
Sourcing PAT Equipment for Cultivated Meat
Finding the right tools for real-time monitoring in cultivated meat production can be tricky. Fortunately, platforms like Cellbase simplify the process. This specialised B2B marketplace focuses exclusively on the cultivated meat industry, connecting buyers with verified suppliers of sensors, probes, and analytical tools tailored to this field. Each listing includes specific use-case tags - such as scaffold-compatible, serum-free, or GMP-compliant - making it easier to identify suitable equipment [5].
Given that growth media often accounts for over 50% of production costs [5], sourcing effective monitoring equipment to optimise nutrient use is not just practical but economically smart. Cellbase also offers global shipping and cold chain options, ensuring temperature-sensitive sensors arrive in perfect condition [5]. For companies transitioning from R&D to commercial production, this streamlined access to compatible equipment reduces risk and speeds up procurement timelines.
sbb-itb-ffee270
Building Predictive Models for Process Optimisation
Once you've deployed PAT tools, the next step is to use predictive models to estimate variables that are difficult to measure directly, such as cell viability and metabolite levels [12]. By analysing spectral data, you can achieve faster and smarter process control. The challenge lies in transforming this data into reliable predictive models.
Developing Chemometric Models
Partial Least Squares Regression (PLSR) is a great starting point for dealing with the overlapping and noisy signals often encountered in cultivated meat production [7][13]. To refine Raman spectra, which can include 1,000–3,000 variables per measurement [7], pre-process the data using derivative calculations. This helps reduce noise while preserving critical peaks. However, be careful not to over-smooth the data, as this could erase the very signals your model depends on.
Variable selection is just as important. Principal Component Analysis (PCA) can help pinpoint which spectral regions are most strongly linked to your target parameter. For instance, a 2018 study revealed that the eighth principal component (PC8) was highly correlated with glucose concentration. The researchers used this insight to fine-tune their PLSR model [7]. This focused approach reduces the risk of overfitting and ensures the model zeroes in on meaningful data.
For cultivated meat production, blending data-driven models with mechanistic ones, like Flux Balance Analysis (FBA), can be particularly effective. In 2023, Oxford Biomedica used a refractometry-based PAT system (the Ranger system) to monitor HEK293T cell cultures. By integrating real-time data with metabolic flux analysis, they uncovered how pH directly influenced intracellular oxygen levels and metabolic activity. This hybrid strategy led to the development of a pH operating plan that boosted metabolic activity by 1.8 times compared to unoptimised processes [12][14].
Once your model is built, the next step is to ensure it performs accurately and reliably under real-world operating conditions.
Validating Models for Production Use
The true test of a model lies in its validation. Start by evaluating it against an independent dataset - data that wasn't part of the training phase. Use metrics like the Root Mean Square Error of Prediction (RMSEP) to gauge its accuracy. For glucose monitoring in cultivated meat processes, prediction errors ranging from 2.39 mM to 6.28 mM are typically acceptable for real-time automated control [7].
Scalability is another key factor. Your model needs to deliver consistent results whether applied in a small R&D bioreactor or a large commercial system. A 2018 study showed that a PLSR model maintained its predictive accuracy when scaled up from a 10 L to a 100 L system [7].
Finally, test the model in dynamic conditions by using "parameter probing." This involves tweaking variables like pH or dissolved oxygen to check if the model tracks changes accurately [14]. Oxford Biomedica used this method to validate an autonomous pH control system [12]. After this, conduct closed-loop tests to confirm the PAT system can maintain parameters within the desired range [14].
Implementing Real-Time Process Control
Real-time process control takes predictive models a step further by using continuous data to maintain optimal performance. By converting live sensor data into automated adjustments, these systems ensure that key conditions like nutrient levels, pH, and dissolved oxygen are consistently regulated - without requiring manual intervention. This not only reduces labour costs and human error but also guarantees a more consistent product quality. For cultivated meat production, such automation is a game-changer in achieving real-time process optimisation.
To make this work, it's crucial to directly measure critical process parameters (CPPs) and feed those signals into your control system. Dan Kopec, a PAT expert at Sartorius Stedim Biotech, highlights the importance of this approach:
The best way to control a critical process parameter (CPP) is to measure that specific parameter, integrate the live signal into your control system, and apply a smart feedback algorithm for an automated control loop. [4]
These feedback loops compare real-time sensor readings against predefined setpoints. Using PID algorithms, they automatically adjust critical parameters like nutrient feeding, pH, and dissolved oxygen to keep everything running smoothly.
For instance, in cultivated meat production, in situ sensors deliver near-instant measurements. Capacitance sensors, for example, can track viable cell volume by treating cells as microcapacitors within a radiofrequency field. This data can then trigger automated cell bleed controls in continuous perfusion processes, helping to maintain steady cell density.[4]
Setting Up Feedback Control Systems
In cultivated meat production, parameters like glucose, pH, and dissolved oxygen directly influence cell growth and metabolic efficiency. Keeping glucose levels low (around 0.1–0.5 g/L) is particularly important to prevent lactate build-up.[4] To address this, Sartorius Stedim Biotech developed the BioPAT Trace system. This technology uses enzymatic biosensors and a dialysis probe with a 10 kDa membrane to provide glucose measurements as frequently as once per minute - without losing volume. This ensures high cell density in perfusion bioreactors.[4]
Automating pH control can also lead to significant improvements. In one study, researchers at Oxford Biomedica and WattBE Innovations used the Ranger Refractive Index (RI) PAT system to monitor HEK293T cell cultures. By developing a 'Metabolic Rate Index' (MRI) and adjusting pH setpoints, they achieved a 1.8-fold increase in metabolic activity. This technique, often referred to as "parameter probing", involves tweaking variables to observe system responses and refine operational conditions.[12]
To enhance reliability further, virtual sensors can act as a backup to hardware sensors. For example, a virtual sensor based on capacitance readings might cross-check glucose data from a Raman probe. This redundancy helps detect sensor drift or failure before it disrupts the process - an especially useful safeguard when dealing with high process variability.
Examples of Real-Time Automation in Cultivated Meat
Real-time control strategies have already delivered impressive results in various applications. For instance, Sartorius Stedim Biotech collaborated with the GSK Medicine Research Centre to use the BioPAT platform for automated closed-loop feeding in CHO cell cultures. This eliminated manual sampling and ensured a steady supply of nutrients.[4]
In another example, Oxford Biomedica integrated the Ranger RI system with metabolic flux analysis to create an autonomous pH control strategy. This system adapted to the cells' metabolic state and detected microbial contamination up to 200 hours earlier than traditional methods, showcasing the potential of real-time monitoring to prevent costly batch failures.[12]
Platforms like Cellbase are also simplifying the procurement process for PAT equipment, making it easier to implement these advanced systems.
As Kopec aptly sums up:
Automation and real‑time monitoring should improve processes with quality and yield gains as well as reductions in labour costs, risk, and waste. [4]
To get started, focus on the most critical parameters - typically glucose, pH, and dissolved oxygen - and gradually expand automation as you gain a deeper understanding of your process. This step-by-step approach is essential for optimising cultivated meat production through real-time control.
Conclusion: Steps to PAT Implementation
Bringing Process Analytical Technology (PAT) into cultivated meat production calls for a clear and methodical approach. Start by identifying your Critical Process Parameters (CPPs) - these could include glucose levels, pH, and dissolved oxygen, all of which have a direct influence on product quality. Once these are defined, choose PAT tools like Raman spectroscopy or capacitance sensors to enable real-time monitoring.
The next step is integrating these sensors into your bioreactor systems and creating predictive models to make sense of the collected data. Prioritise in-line monitoring whenever possible, as it eliminates delays and lowers the risk of contamination during the process.
Automated feedback systems play a crucial role here, converting raw data into immediate, actionable adjustments. As Sigma-Aldrich aptly puts it:
A key objective of PAT is to build quality into products rather than evaluating quality at the end of the process. [6]
This proactive approach not only reduces labour costs but also ensures consistent product quality while cutting down on waste.
Once automated feedback systems are operational, the next focus should be on sourcing the right PAT equipment. Reliable equipment is vital for success, and platforms like Cellbase specialise in providing vetted sensors and monitoring tools tailored for cultivated meat production. They also offer technical support for calibration and seamless system integration [5]. This ensures that the equipment aligns perfectly with the unique demands of cultivated meat production.
As your understanding of the process deepens, gradually expand automation to achieve scalable and consistent production while meeting regulatory standards. By following these steps, PAT implementation can become the backbone of a more efficient and reliable cultivated meat production process.
FAQs
What are the benefits of using Process Analytical Technology (PAT) in cultivated meat production?
Process Analytical Technology (PAT) plays a key role in improving both process control and product consistency in cultivated meat production. With real-time monitoring of crucial factors like temperature, pH levels, and dissolved oxygen, PAT ensures optimal growth conditions for cells while minimising the chances of unexpected process issues. The result? Higher yields, consistent quality, and reduced production costs.
Another advantage of PAT is how it supports a Quality-by-Design (QbD) framework. By directly connecting analytical data to the specific quality characteristics of the product, it reduces dependence on traditional end-point testing methods. This approach not only speeds up validation processes but also enables data-driven decisions that improve reproducibility and allow for predictive control strategies.
For companies in the cultivated meat space, platforms such as Cellbase make it easier to source PAT-compatible tools, including sensors and bioreactors. These resources simplify procurement, making it more straightforward to scale up production while maintaining efficiency.
How does Raman spectroscopy improve real-time monitoring in PAT systems for cultivated meat production?
Raman spectroscopy plays a crucial role in real-time monitoring within PAT (Process Analytical Technology) systems by delivering quick, non-invasive, inline measurements of key process parameters. This helps maintain tighter process control and ensures consistent product quality throughout.
One of its standout features is the ability to detect multiple molecules simultaneously. For instance, it can monitor glucose, lactate, and ammonium levels while also assessing cell viability and product characteristics - all in a single measurement. Modern Raman probes are designed to be installed directly into bioreactor streams, allowing for continuous data collection without the need to extract samples.
Another advantage is its support for automated feedback control. By providing real-time data, Raman spectroscopy enables precise adjustments to nutrient feeds, ensuring optimal production conditions are maintained. Its flexibility in scaling and transferring models across various reactor sizes further enhances its utility in cultivated meat production, boosting efficiency and minimising the risk of errors.
What are the key challenges in scaling up Process Analytical Technology (PAT) for cultivated meat production?
Scaling up PAT (Process Analytical Technology) for large-scale cultivated meat production comes with its fair share of hurdles, demanding meticulous planning and execution. A key issue lies in managing and integrating the enormous volumes of data generated by PAT instruments. As production scales up, maintaining data accuracy while ensuring smooth integration into control systems becomes a more intricate task.
Another significant obstacle is the performance of sensors in industrial-scale bioreactors. Sensors that work well in smaller setups often face challenges in larger systems, where factors like shear forces and temperature variations can compromise the accuracy of real-time measurements.
There's also the issue of procuring specialised equipment tailored to the unique demands of cultivated meat production. Platforms such as Cellbase help tackle this by offering a curated marketplace for food-grade sensors and analysers, easing supply-chain constraints and simplifying equipment sourcing.
Tackling these challenges early - by selecting reliable sensors, building scalable data systems, and planning procurement strategically - can help businesses navigate the shift to commercial-scale production more efficiently.











