Contamination is a major hurdle in cultivated meat production, with batch failure rates hitting 11.2% and climbing to 19.5% in larger-scale operations. This not only wastes resources like growth media (over 50% of production costs) but also disrupts timelines. Effective decontamination is key to minimising these risks. Here's a quick overview of the top tools used to maintain sterility in cultivated meat facilities:
- Industrial-Grade Detergents and Degreasers: Remove organic residues like fats and proteins, essential for pre-sanitisation cleaning.
- Food-Grade Sanitisers: Reduce microbial loads after cleaning, targeting bacteria and biofilms.
- Clean-in-Place (CIP) Systems: Automate internal cleaning of bioreactors and pipelines without disassembly.
- UV Decontamination Lamps: Use UV-C light to disinfect surfaces and air without chemicals.
- Hydrogen Peroxide Vapour Generators: Provide thorough, touchless sterilisation for rooms and equipment.
- Stainless Steel Disinfection Wardrobes: Sanitize tools, PPE, and small equipment in a controlled environment.
- Automated Sensor Cleaning Stations: Keep bioreactor probes clean and functional to maintain accurate monitoring.
Each tool addresses specific contamination challenges, from cleaning surfaces to sterilising equipment and maintaining biosafety standards. Combining these methods ensures safer, more efficient production while reducing costly failures. Below, we dive into how each tool works and its practical applications in cultivated meat production.
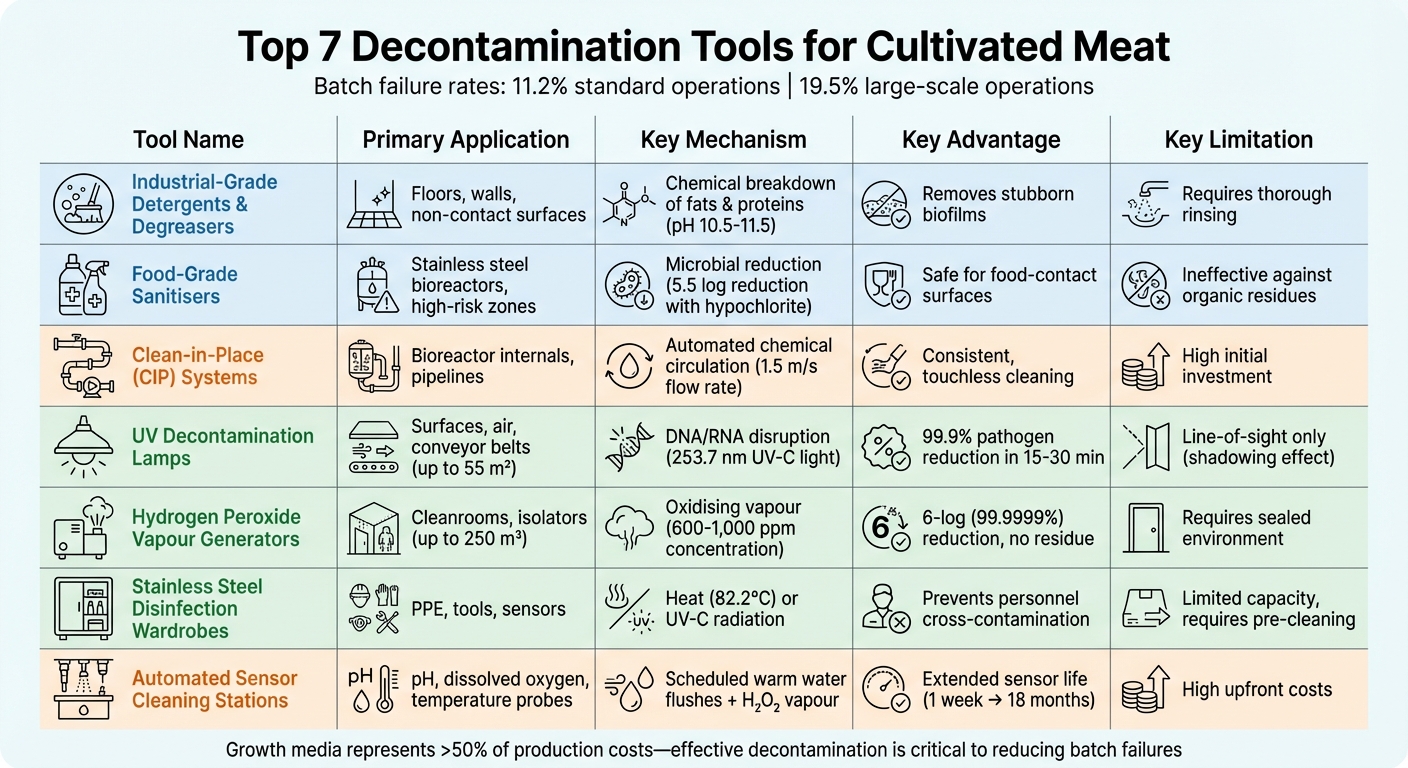
Comparison of 7 Decontamination Tools for Cultivated Meat Production
1. Industrial-Grade Detergents and Degreasers
Industrial-grade detergents and degreasers play a crucial role in maintaining cleanliness in cultivated meat production facilities. These powerful cleaning agents are designed to physically remove organic residues - such as fats, proteins, and cellular debris - that build up on surfaces and equipment during production. Skipping this essential cleaning step can undermine sanitisation efforts, as leftover organic matter can shield bacteria from disinfectants.
After the initial cleaning, specific applications are used to improve the overall decontamination process.
Primary Application
Alkaline detergents with a pH range of 10.5–11.5 (containing at least 200 ppm active alkalinity and 200 ppm chlorine) are highly effective at breaking down organic soils. Acidic compounds, on the other hand, are used to remove mineral deposits lodged in equipment crevices [7]. For vertical surfaces, high-foaming chlorinated cleaners are preferred, as their extended contact time - typically 15 minutes - ensures thorough cleaning [6].
Decontamination Method
Cleaning begins with warm water (<48.9°C) to rinse surfaces, followed by manual scrubbing to disrupt biofilms. For Clean-in-Place (CIP) systems, low-foaming caustic cleaners are recommended to avoid issues like pump cavitation [5][8]. Once detergents are applied, a complete rinse with potable water is essential. This step is critical because most detergents are alkaline, while many sanitisers are acidic - any leftover detergent can neutralise the sanitiser, rendering it ineffective [8].
Compatibility with Cultivated Meat Equipment
Material compatibility is another key consideration. Chlorinated products, for instance, can cause premature wear and tear on rubber or silicone components, such as those found in bioreactor seals and tubing [7]. For delicate equipment like bioreactor filters, fume hoods, or 316-grade stainless steel tanks, specialised degreasers are used to remove hardened grease without damaging sensitive surfaces [4]. Non-foaming alkaline degreasers are also ideal for deep cleaning large areas, such as floors and walls, using industrial scrubber machines [4].
Advantages and Limitations
While detergents are effective at removing organic matter that promotes bacterial growth, they do not kill resilient bacteria like Salmonella and E. coli [8]. This limitation highlights the need for a two-step process: cleaning followed by sanitisation. Factors like water quality, including pH and hardness, can also influence detergent performance. In dry processing environments, traditional wet detergents may not be suitable, as excess moisture can lead to mould growth. Additionally, following the manufacturer’s dilution guidelines is critical - diluting too much can reduce effectiveness, while overly concentrated solutions can damage equipment and compromise product safety [8].
For professionals in the cultivated meat industry, these essential cleaning agents are available on Cellbase, a dedicated B2B marketplace tailored to meet industry needs.
2. Food-Grade Sanitisers
After cleaning with detergents, food-grade sanitisers play a crucial role in reducing microorganisms to safe levels. These chemical agents are particularly effective against bacteria that form biofilms, which act as protective barriers for harmful pathogens like L. monocytogenes. A study conducted across 23 food processing facilities revealed that 65% of them tested positive for Listeria even after cleaning and sanitisation protocols were completed [9].
The effectiveness of sanitisers depends largely on thorough pre-cleaning. When protein residues are left behind on surfaces, their performance can drop significantly. For instance, hypochlorite solutions, which typically achieve a 5.5 log reduction, see their effectiveness plummet to just 2.8 in the presence of organic matter [9]. By first removing organic residues, sanitisers can then work effectively to eliminate remaining microorganisms.
Primary Application
Sanitisers are indispensable after chemical cleaning, particularly in cultivated meat production environments. Peroxyacetic acid (PAA) is especially effective for sanitising stainless steel bioreactor surfaces. Meanwhile, alcohol-based sanitisers are ideal for areas sensitive to moisture, where traditional wet cleaning might encourage mould growth. High-risk zones such as drains and slicing areas require focused sanitisation to address persistent contamination hotspots [8][9].
Decontamination Method
How sanitisers are applied greatly affects their performance. Direct application or foaming methods provide better disinfection compared to fogging [9]. After cleaning with detergents, a thorough rinse is essential, as detergents are often alkaline and can neutralise the typically acidic sanitisers. It’s also critical to use sanitisers at the manufacturer-recommended dilutions. Over-diluting can lead to bacterial tolerance, while overly concentrated solutions risk damaging equipment or contaminating products [8]. These steps ensure effective sanitisation across all equipment used in cultivated meat production.
Compatibility with Cultivated Meat Equipment
Food-grade sanitisers are generally compatible with the stainless steel and ceramic surfaces commonly used in cultivated meat facilities. Quaternary ammonium compounds can achieve a 6.1 log reduction on properly cleaned surfaces, although some bacterial strains have developed resistance through plasmids. On the other hand, PAA is highly effective at penetrating biofilms, making it an excellent choice for sanitising bioreactor components [9].
Advantages and Limitations
While sanitisers are excellent at reducing bacterial loads to safe levels, they are not a substitute for proper cleaning. Organic residues can shield bacteria, significantly reducing the effectiveness of these chemicals. Additionally, bacteria exposed to sanitisers may become viable but undetectable, creating hidden risks. In wet processing areas, it’s advisable to use fans to dry surfaces daily after sanitisation, preventing the growth of moisture-loving bacteria [9]. When used correctly, these sanitisers complement the cleaning tools discussed earlier, forming a vital part of the step-by-step decontamination process required to uphold biosafety standards.
For those sourcing decontamination products, Cellbase offers verified food-grade sanitisers tailored to the specific needs of cultivated meat production.
3. Clean-in-Place (CIP) Systems
Clean-in-Place (CIP) systems automate the cleaning of enclosed production equipment, eliminating the need for disassembly or manual scrubbing. These systems circulate chemical detergents through bioreactors, tanks, piping, and heat exchangers at specific temperatures and flow rates. This creates a turbulent "scrubbing" effect that efficiently removes residues from internal surfaces, helping to minimise contamination risks and reduce downtime in cultivated meat production facilities [12].
Primary Application
CIP systems are indispensable for large-scale bioprocessing equipment used in cultivated meat production, such as fermenters, centrifugal separators, and filter housings [12]. They are particularly useful for equipment that is too large or complex to clean manually. Once the CIP process is complete, facilities typically follow up with Sterilise-in-Place (SIP) procedures to ensure aseptic conditions [10]. This step-by-step approach ensures thorough cleaning and sterilisation.
Decontamination Method
The CIP process follows a carefully validated sequence: pre-rinse, caustic wash (to break down proteins and fats), intermediate rinse, acid rinse (to remove mineral deposits), sanitisation, and a final post-rinse [12][15]. For effective cleaning, parameters like temperature, flow, pressure, chemical concentration, and contact time must be optimised. For instance, pipelines need a flow rate of at least 1.5 m/s to achieve proper scrubbing [12]. Static spray balls, commonly used in these systems, operate at 90–136 L/min with a pressure drop of 1.4–2.1 bar, effectively cleaning a diameter of up to 2.4 m [12].
"The process jets cleaning solutions over surfaces under high turbulence and flow." - Society of Dairy Technology [11]
Compatibility with Cultivated Meat Equipment
CIP systems work particularly well with the stainless steel surfaces found in cultivated meat facilities. However, timing is crucial - cleaning chemicals or sanitisers need to be rinsed off within 20 minutes to prevent pitting or corrosion [12]. Equipment design also plays a key role in CIP effectiveness. For instance, designs should avoid "dead legs" (areas where fluid doesn't circulate) and ensure smooth, high-quality welds, as rough joints can trap contaminants that CIP systems cannot reach [10][12]. Riboflavin dye tests are commonly used to verify the coverage of spray devices. The dye fluoresces under UV light, highlighting any areas that were missed during cleaning [12]. These measures are essential for maintaining the sterile conditions required in cultivated meat production.
Advantages and Limitations
CIP systems provide consistent, reliable cleaning results with every cycle, reducing human exposure to high temperatures and harsh chemicals [11][12]. They also minimise equipment downtime and offer automated digital records to meet regulatory requirements [11]. On the downside, CIP systems require significant initial investment, precise control of cleaning parameters, and ongoing maintenance to address issues like clogged spray heads or gasket wear [12]. Modern CIP systems are increasingly designed with reuse capabilities, allowing cleaning fluids to be reclaimed and stored. This approach reduces water, chemical, and energy consumption compared to single-use systems [10][12].
For cultivated meat companies, sourcing CIP-compatible equipment is essential. Cellbase connects production facilities with trusted suppliers of bioprocessing systems tailored for automated cleaning protocols.
4. UV Decontamination Lamps
UV-C decontamination lamps operate by emitting ultraviolet light within the 200–280 nm range. This light sterilises surfaces and air without the need for heat or chemicals, making it a key tool in cultivated meat facilities. These environments require strict sterility to avoid chemical residues that could disrupt cell culture processes. The lamps work by targeting the DNA and RNA of microorganisms, rendering them inactive [16][18].
Primary Application
UV-C lamps are primarily used for zonal transfer, ensuring equipment and materials are decontaminated as they move into high-care areas like bioreactor rooms [16]. Beyond this, they are effective for disinfecting conveyor belts, cutting tools, machine surfaces, and packaging materials [19]. Industrial-grade mobile units can sanitise areas up to 55 square metres, achieving a 99.9% pathogen reduction in just 15 to 30 minutes [17]. This speed is particularly important in cultivated meat production, where maintaining sterile conditions while keeping to tight schedules is crucial.
Decontamination Method
The germicidal process is simple: UV-C light at 253.7 nm is absorbed by microbial DNA, altering its structure and stopping replication [16][17]. This method works against a broad spectrum of microorganisms, including bacteria like Listeria and Salmonella, viruses such as SARS-CoV-2, and even yeasts, moulds, and spores [16][18]. However, the effectiveness of UV-C is limited to what the light can directly reach.
"As it's light-based, UV-C systems must be able to 'see' the organisms to inactivate them. So it goes without saying that shadows and shields dramatically reduce this technology's effectiveness." - Danny Bayliss, New Technologies Lead, Campden BRI [16]
For optimal results, surfaces need to be smooth and fully exposed, as textured areas can create pockets where pathogens remain shielded [16]. Additionally, UV-C systems are designed with safety in mind, often featuring delay-start timers and motion sensors to ensure no humans, pets, or plants are present during operation [17]. These factors highlight UV-C as one component of a broader decontamination strategy in cultivated meat facilities.
Compatibility with Cultivated Meat Equipment
UV-C lamps are particularly well-suited to the stainless steel and food-grade plastics commonly used in cultivated meat production [16][19]. Their non-thermal, chemical-free operation ensures sensitive equipment remains undamaged while avoiding contamination of cell cultures [18][19]. Options range from compact tabletop units, priced between £210 and £230, to larger mobile carts costing around £950 [17]. Facilities employing UV-C systems for zonal transfers must validate their processes to meet standards like the BRCGS Global Standard for Food Safety [16]. This compatibility makes UV-C an integral part of maintaining sterility in cultivated meat production.
Advantages and Limitations
UV-C lamps offer several benefits, including rapid and residue-free decontamination. They can eliminate up to 99.99% of microorganisms in seconds, without leaving moisture or chemicals behind [19]. This makes them ideal for heat-sensitive materials that cannot withstand thermal sterilisation [18]. However, their reliance on direct exposure means they struggle with complex equipment that has hidden crevices [16]. Different microorganisms also vary in their susceptibility to UV light, so facilities must validate their systems against the specific pathogens they aim to control [16].
5. Hydrogen Peroxide Vapour Generators
Hydrogen peroxide vapour (HPV) generators are devices designed to quickly convert a 35% hydrogen peroxide solution into vapour. This vapour then condenses evenly across surfaces, ensuring thorough coverage [23][25]. In cultivated meat facilities, these systems play a key role in decontaminating areas like cleanrooms, isolators, transfer hatches, and enclosed equipment such as incubators and freeze dryers [20][22]. One particularly important use is restoring aseptic environments after maintenance work - such as when equipment panels are opened - since such activities can introduce spores into otherwise sterile spaces [23]. HPV generators complement other automated cleaning methods by effectively targeting areas that manual cleaning might miss.
Primary Application
HPV generators are especially useful for reaching tricky spots that manual cleaning often overlooks, such as wiring conduits, sensors, and intricate bioreactor components [23]. Modern portable units, like the Bioquell L-4, can effectively decontaminate spaces as large as 250 cubic metres when fitted with a distribution head [22]. A study conducted between February 2021 and January 2024 found that applying HPV after maintenance helped maintain stable microbial counts, outperforming manual cleaning methods [23].
Decontamination Method
The decontamination process with HPV involves four key phases:
- Dehumidification: Reducing humidity to a range of 5–40%.
- Conditioning: Introducing the hydrogen peroxide vapour.
- Bio-decontamination: Sustaining a vapour concentration of 600–1,000 ppm.
- Aeration: Breaking the vapour down into water and oxygen via catalytic conversion [20].
The vapour works as a powerful oxidising agent, disrupting microbial DNA, proteins, and lipids, achieving a 6-log (99.9999%) reduction in pathogens, including highly resistant bacterial spores [20][21]. To ensure the process is effective, facilities typically use Geobacillus stearothermophilus endospores, which are considered the industry benchmark for testing HPV resistance [23].
"Hydrogen peroxide vapor generators provide touchless decontamination that can circumvent problems associated with operators such as incorrect application of cleaning agents during manual disinfection procedures." - Tim Sandle, Head of GxP Compliance and Quality Risk Management, Bio Products Laboratory [23]
Compatibility with Cultivated Meat Equipment
One of the standout features of HPV is its ability to operate at low temperatures, making it ideal for decontaminating heat-sensitive equipment used in cultivated meat production [20][23]. Additionally, the vapour naturally breaks down into water vapour and oxygen, leaving no toxic residues behind. This eliminates the need for post-cleaning wipe-downs, which is particularly important in cultivated meat facilities where chemical residues could interfere with delicate cell cultures [20][23]. Some systems also integrate with Building Management Systems via Modbus TCP/IP, allowing for automated data collection and cycle validation [22].
Advantages and Limitations
HPV excels at reaching intricate shapes and crevices and is compatible with materials like stainless steel and sensitive electronics [20][24]. However, it does have its limitations. As a surface-contact agent, it cannot penetrate porous materials or areas that are physically blocked [23]. European medicines inspectors have noted that the effectiveness of HPV cycles can be sensitive to variables like gas concentration, exposure time, temperature, and humidity [23]. Additionally, adequate aeration time is essential before personnel can safely re-enter treated spaces, as the vapour remains hazardous during the active cycle [22].
sbb-itb-ffee270
6. Stainless Steel Disinfection Wardrobes
Stainless steel disinfection wardrobes create a controlled space to sanitise high-contact tools and PPE, such as processing trays, utensils, sensors, face shields, masks, and gloves. These items can harbour harmful pathogens like Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes [27][28][29]. In cultivated meat production, where maintaining sterile conditions is critical for cell culture success, these wardrobes serve as a key checkpoint to prevent cross-contamination between personnel and the product [13].
Primary Application
These wardrobes are particularly useful for managing the movement of materials between quarantine areas and tissue culture zones [26]. They are also indispensable for sanitising delicate bioprocessing sensors, which require real-time data collection but are unsuitable for high-pressure wet cleaning methods [3]. The importance of such tools is highlighted by the U.S. Food Safety Inspection Service, which has the authority to halt production if sanitation standards are not met [13].
Decontamination Method
Stainless steel disinfection wardrobes typically use heat or UV light to kill microorganisms. For effective microbial reduction, water used in these systems should reach at least 82.2°C [13][14][15]. Pre-cleaning is essential to remove debris, as leftover organic material can cause proteins to bond permanently to the stainless steel surface [14]. Additionally, peracetic acid has been shown to reduce E. coli and Salmonella by 1.5–5.8 log CFU, depending on its concentration and exposure time [29].
Compatibility with Cultivated Meat Equipment
These wardrobes integrate seamlessly with materials commonly used in cultivated meat production. For example, stainless steel stirred-tank bioreactors - designed for animal cell production at scales of up to 20,000 litres - are built to endure frequent and rigorous sterilisation [30]. The wardrobes also offer a safe environment for rust-resistant tools and sensitive monitoring devices that cannot withstand high-pressure steam cleaning [3].
Advantages and Limitations
One major advantage of stainless steel disinfection wardrobes is their ability to deliver consistent and organised sanitisation for small tools that might otherwise be overlooked during general cleaning routines. They also protect stainless steel items from the corrosive effects of industrial degreasers, reducing the risk of human contamination in cleanroom-like environments [13]. However, these systems are not without limitations. Shadowed areas can remain unsanitised if items are poorly arranged [14]. Additionally, the pre-cleaning step adds extra effort, and only potable water can be used, as non-potable water is strictly prohibited in areas where it might come into contact with edible products [14].
For those in the industry, specialised wardrobes like these are listed on Cellbase for easy access.
7. Automated Sensor Cleaning Stations
Automated sensor cleaning stations play a vital role in keeping probes like pH, dissolved oxygen, and temperature sensors clean and functioning accurately. In the world of cultivated meat production, even small shifts in these parameters can result in lower yields, contamination, or wasted resources [1]. These stations not only cut down on manual cleaning but also help maintain sterility, minimising contamination risks while supporting the closed systems crucial for cell culture [3].
Primary Application
These stations build on automated decontamination processes and integrate directly into monitoring systems. They deliver real-time information on critical parameters such as cell density, viability, and metabolic activity [3][31]. By automating cleaning and calibration, they allow for longer culture durations, enable predictive controls, and ensure data logging for regulatory purposes [3]. For instance, an industrial system using automated flushing extended the lifespan of a pH sensor from just one week to 18 months by preventing the accumulation of solids, fats, and proteins [33].
Decontamination Method
These systems rely on scheduled warm water flushes and, when needed, hydrogen peroxide vapour to prevent sensor fouling [33][32]. It’s important to avoid directly spraying disinfectants like 70% ethanol into sensor openings; instead, sensors should be wiped with a damp, non-woven cloth [32]. Warm water flushes are particularly effective for removing waxy or fatty residues that often build up during cultivated meat production [33].
Compatibility with Cultivated Meat Equipment
Automated cleaning stations are designed to seamlessly integrate with standard bioreactor and incubation systems, often including technical support for calibration and system setup [3][31]. They work with a variety of sensors essential to cultivated meat production, including those for pH, dissolved oxygen, ozone, and hydrogen peroxide [33]. Additionally, non-invasive monitoring technologies allow for continuous data collection without compromising the sterile environment.
Advantages and Limitations
These stations bring several benefits: they lower labour costs, reduce human error, and extend the lifespan of equipment through consistent maintenance [33][34].
"Automated equipment follows pre-programmed routines that ensure all surfaces are cleaned to specification, every time." - Kelly Gavson, Director of Finance at FOG Tank [34]
They also improve worker safety by limiting exposure to harsh chemicals and high-pressure sprays. However, they do come with challenges, such as high upfront costs and the need for periodic manual calibration [33][35]. To optimise their use, flush parameters should be tailored to the specific fouling characteristics of the culture media, balancing cleanliness with water efficiency [33]. These automated systems are a key component in maintaining strict biosafety protocols across facilities.
For cultivated meat facilities looking for tailored solutions, companies like Cellbase offer sensor cleaning stations designed to meet specific monitoring needs.
Tool Comparison Table
Here’s a detailed comparison of various decontamination tools, outlining their applications, cleaning methods, compatibility, benefits, and limitations.
| Decontamination Tool | Primary Application | Cleaning Mechanism | Equipment Compatibility | Advantages | Limitations |
|---|---|---|---|---|---|
| Industrial-Grade Detergents and Degreasers | Floors, walls, and non-contact surfaces | Chemical breakdown of organic matter | Epoxy floors, stainless steel, PVC, ceramics, rubbers | Effectively removes stubborn biofilms and fats; suitable for machine cleaning | Requires thorough rinsing to avoid cell toxicity; involves strict rinsing protocols |
| Food-Grade Sanitisers | Workbenches, tools, centrifuges, food-contact surfaces | Microbial inactivation (e.g., 70% ethanol) | Most non-porous surfaces | Safe for food-contact surfaces; poses lower toxicity risks | Less effective against hardy contaminants; may not eliminate all bacterial spores |
| Clean-in-Place (CIP) Systems | Bioreactor internals, piping | Automated chemical/heat circulation | Stainless steel closed-loop systems | Reduces manual handling risks; ensures consistent sterilisation of internal surfaces | High initial costs; complex design and installation requirements |
| UV Decontamination Lamps | Air and surface (biosafety cabinets, clean rooms) | DNA/RNA disruption via UVC light | Laminar flow hoods; clean rooms | Chemical-free; easy to automate; provides broad-spectrum microbial control | Limited to line-of-sight cleaning (shadowing effect); prolonged use may degrade certain plastics |
| Hydrogen Peroxide Vapour Generators | Whole-room sterilisation; large equipment | Oxidising hydrogen peroxide vapour | Sealed rooms; BSL-3/4 facilities | Highly effective against spores; breaks down into water and oxygen; leaves no toxic residues | Requires sealed environments and evacuation during use; lengthy sterilisation cycles |
| Stainless Steel Disinfection Wardrobes | PPE, lab coats, and small tools | UV-C radiation or ozone | Fabrics; stainless steel tools | Targets contamination from personnel; helps maintain ISO Class 8 environments | Limited capacity; requires careful loading; lower throughput |
| Automated Sensor Cleaning Stations | Bioreactor probes (pH, dissolved oxygen) | Automated rinsing and sterilisation | Standard bioreactor and incubation systems | Reduces contamination risks during sampling; extends sensor lifespan; lowers labour costs | High initial investment; periodic manual calibration is necessary |
This table highlights essential features of decontamination tools, helping facilities align their choices with operational and budgetary needs. By combining physical and chemical methods, contamination rates can be effectively minimised, ensuring food-grade standards are upheld for commercial production [28].
For tailored solutions, cultivated meat facilities can explore verified decontamination tools available on Cellbase to meet specific production requirements.
Conclusion
Ensuring effective decontamination is absolutely essential for the success of cultivated meat production. As Cellbase highlights, maintaining sterility in bioreactors is non-negotiable - contamination not only destroys batches but also wastes valuable resources and disrupts production timelines [3]. Considering that growth media accounts for over 50% of total production costs, even a single contaminated batch can lead to significant financial setbacks [1]. This makes a multi-layered approach to decontamination a necessity.
A well-rounded biosafety strategy combines various tools to tackle contamination risks from multiple angles. Industrial-grade detergents, food-grade sanitisers, CIP systems, UV lamps, hydrogen peroxide vapour generators, disinfection wardrobes, and automated sensor cleaning stations all play specific roles in ensuring sterility. However, their effectiveness depends on proper validation and sequencing - cleaning must always come before sanitisation [8]. Additionally, facilities need to ensure that all chemicals used are certified by third-party programmes like NSF, confirming their suitability for food-contact surfaces [8].
The industry is also moving towards automation and closed systems as part of a broader trend. A notable example is the CelCradle® +, launched in January 2025 by Esco Aster and Esco Lifesciences Group. This closed, single-use bioreactor system meets stringent BSL 3/4 standards and is designed to replace manual roller bottle technology with a scalable, automated alternative [2]. This innovation highlights how advanced decontamination and containment technologies are becoming indispensable for large-scale commercial production.
FAQs
How can decontamination tools help prevent batch failures in cultivated meat production?
Decontamination tools like autoclaves, chemical disinfectants, UV sterilisers, and clean-in-place (CIP) systems are essential for keeping microbial contamination at bay in cultivated meat production. These tools ensure that bioreactors, ports, gas filters, and other equipment are sterilised before each production cycle, wiping out bacteria, fungi, and biofilms that thrive in nutrient-rich growth media. This process is critical in reducing the risk of batch contamination, which can lead to expensive production failures.
Contamination isn't just inconvenient - it's costly. Industry statistics reveal an average failure rate of 11.2% due to sterility issues. Implementing effective decontamination methods, such as automated UV surface cleaning, validated autoclave procedures, and CIP systems for continuous cleaning, helps facilities maintain sterility standards. This not only minimises product loss but also ensures consistent results, making it easier to scale up production efficiently.
If you're in the market for reliable decontamination equipment, Cellbase provides a specialised marketplace. They connect professionals in the cultivated meat industry with trusted suppliers offering autoclaves, UV cabinets, CIP modules, and advanced cleaning agents, helping you secure the tools you need to maintain sterility and optimise production.
What are the benefits of using UV-C lamps for decontamination in cultivated meat facilities?
UV-C lamps offer a highly efficient, chemical-free way to sanitise both surfaces and air in cultivated meat production facilities. By disrupting the DNA of harmful microorganisms, they can eliminate up to 99.99% of bacteria, viruses, moulds, yeasts, and spores, ensuring a superior level of cleanliness without relying on harsh chemicals.
What’s more, UV-C lamps don’t produce heat, making them ideal for environments where temperature control is crucial. They’re also easy to maintain and budget-friendly, which makes them a smart solution for keeping production areas clean and safe.
Why is a two-step process of cleaning and sanitisation essential in cultivated meat production?
In cultivated meat production, maintaining safety and hygiene is non-negotiable, and a two-step process of cleaning and sanitisation is central to achieving this.
The first step, cleaning, focuses on removing organic residues and biofilms that could shelter harmful microbes. Once surfaces and equipment are free from these residues, sanitisation comes into play. This step is designed to significantly reduce bacterial loads to levels deemed safe, ensuring the environment is ready for production.
By adhering to this method, facilities not only lower the risk of contamination but also uphold the integrity of their processes and stay aligned with food safety regulations.











