Maintaining precise pH and temperature is critical for growing mammalian cells, especially in cultivated meat production. Cells need a controlled environment to multiply (proliferation) and develop into muscle fibres (differentiation). Here's the key takeaway:
- Optimal Conditions: pH must stay between 7.2–7.4, and temperature at 37 °C. Even small deviations (e.g., pH dropping by 0.3 units) can slow growth and reduce productivity.
- Why It Matters: Cells expend extra energy correcting imbalances, which impacts their growth efficiency. High-density cultures are especially prone to pH drops due to lactic acid build-up.
- Challenges at Scale: Larger bioreactors face uneven conditions, like pH spikes or CO₂ build-up, making precise control harder.
- Solutions: Advanced bioreactors with automated systems and reliable sensors help maintain stability, improving cell growth and consistency.
Whether you're growing cells in a lab or scaling up for production, keeping pH and temperature stable is non-negotiable for success.
Sensors in bioreactors
How pH and Temperature Affect Cell Growth
The roles of pH and temperature in bioreactor design go beyond theoretical importance - they directly influence cell metabolism and growth. This section explores how these two factors shape cellular behaviour and productivity.
pH Effects on Cell Metabolism and Viability
When pH levels deviate from optimal ranges, cells must work harder to maintain balance. For instance, they activate mechanisms like Na⁺/H⁺ antiporters, which consume energy that would otherwise fuel growth [3]. This energy redirection can lead to major shifts in gene activity. In one study, lowering the medium's pH to 6.7 caused over 2,000 genes to change their expression levels within just 24 hours [3].
The interplay between pH and metabolism can create a vicious cycle. High glycolytic activity generates lactic acid, which lowers the medium's pH. In some high-density cultures, up to 90% of glucose is converted into lactate [2], leading to rapid acidification. While this acidification eventually halts further lactic acid production, it comes at the cost of significantly reduced cell growth [5].
Both acidic and alkaline extremes are harmful. While acidic conditions below pH 7.1 are widely known to hinder growth, alkaline conditions - ranging from pH 7.7 to 9.0 - can also slow proliferation and reduce product yields [2][4]. For most mammalian cells, the critical lower pH limit is between 6.6 and 6.8. Beyond this range, cells face heightened risks of apoptosis or necrosis [5].
These pH-driven metabolic disruptions set the stage for temperature's role in further influencing cell behaviour.
Temperature Effects on Cell Proliferation and Differentiation
Temperature plays a pivotal role in metabolic activity and gas solubility. While 37 °C is the standard for most cultures, even slight deviations can impact growth and protein production [3][5]. A study conducted at the Vienna University of Technology in 2017 demonstrated this effect. Researchers used CHO cells in a 10–12 m³ stirred tank bioreactor to simulate pH inhomogeneities. Temporary exposure to pH 9.0 zones during the exponential growth phase significantly reduced both the maximum viable cell density and the final product yield [4].
In the field of cultivated meat production, temperature control serves dual purposes. During the proliferation phase, maintaining a steady 37 °C ensures efficient cell multiplication. However, advanced temperature-responsive systems have been developed to facilitate scaffold-free tissue layering by controlling cell adhesion and detachment [6].
Clearly, precise temperature regulation is just as crucial as maintaining an optimal pH for successful cell growth and differentiation.
Combined pH and Temperature Effects
The interaction between pH and temperature is closely tied to CO₂ chemistry. Changes in temperature affect CO₂ solubility, which in turn influences the acid-base balance in bicarbonate-buffered systems [3]. Higher temperatures speed up cellular metabolism, increasing the production of by-products like lactic acid and CO₂. This further acidifies the medium, compounding the stress on cells [2][3].
"The solubility of dissolved gases, and thus the influence of CO₂ on acid-base chemistry, is strongly dependent on temperature, osmolarity, humidity, and pressure." - Shannon G. Klein et al., King Abdullah University of Science and Technology [3]
When pH and temperature deviate simultaneously, the resulting metabolic stress can severely disrupt both cell proliferation and differentiation. For example, standard batch cultures often show a median pH shift of 0.425 units [3]. In high-density cultures, this shift can reach 0.9 units, accompanied by CO₂ levels rising to 10.45% [3]. These conditions force cells to expend even more energy on maintaining homeostasis, reducing their efficiency in biomass production.
To minimise these stresses, freshly prepared media should be equilibrated in a CO₂ incubator for at least an hour before use. This allows the slow reverse reaction of CO₂ hydration to stabilise [2]. Such precautions are essential for achieving optimal cell growth and productivity.
Methods for Controlling pH and Temperature in Bioreactors
Keeping pH and temperature stable in bioreactors involves a mix of hardware, sensors, and control strategies. The technology chosen often depends on the production scale, the type of cells being used for cultivated meat, and whether the process leans more towards automation or manual management.
Bioreactor Design and Control Methods
Bioreactors used in cultivated meat production rely on heat exchange systems to maintain a temperature of 37 °C [1]. pH levels are typically regulated through CO₂ sparging, which adjusts the CO₂ concentration and headspace flow [9], or by automated syringe pumps that add acids or bases as needed [8].
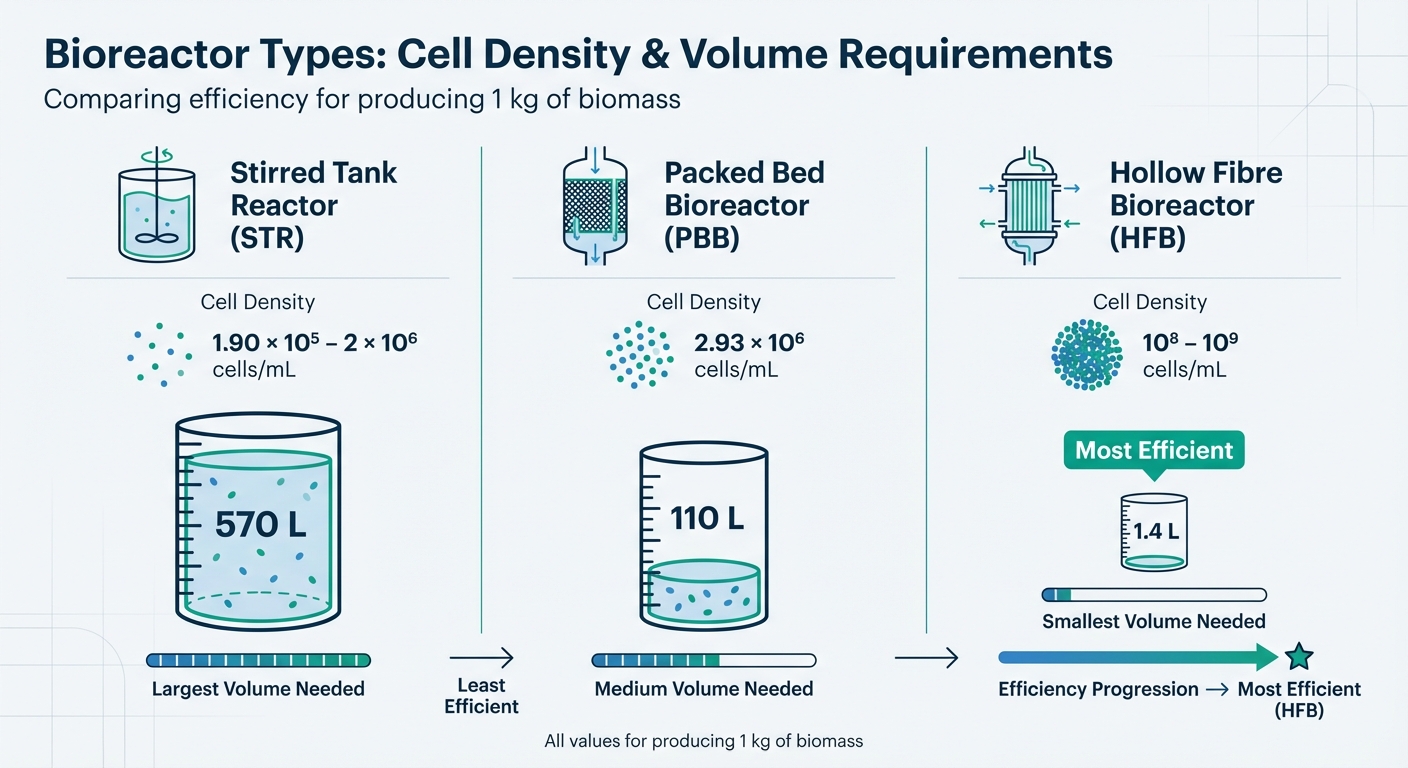
Single-use bioreactors (SUBs) offer a practical solution by eliminating the need for cleaning and reducing contamination risks. These systems can scale up to 2,000 L. However, the working volumes required for producing 1 kg of biomass vary significantly depending on the bioreactor design: approximately 570 L for stirred tank reactors (STRs), 110 L for packed bed bioreactors (PBBs), and just 1.4 L for hollow fibre bioreactors (HFBs) [1].
Sensor Technologies for Monitoring
Once the bioreactor is set up, precise sensors play a critical role in monitoring pH and temperature in real time. For pH measurement, electrochemical sensors, particularly glass electrodes, are widely used due to their durability and effectiveness [7]. When it comes to temperature, resistance thermometers are the industry standard [7].
In recent years, optical sensors have gained popularity, especially in single-use systems. These sensors utilise fluorescent dyes - such as 6,8-dihydroxypyrene-1,3-disulfonic acid disodium salt - embedded in hydrogel patches. They are compact and help minimise contamination risks [7].
Non-contact sensors are another option, using permeable membranes like cellulose to measure pH externally, which further reduces contamination risks [7]. Meanwhile, colourimetric systems track pH by detecting colour changes in phenol red indicators within the media. These systems use LED light sources and ambient light sensors for detection [8]. While optical sensors are less invasive, they can sometimes be affected by issues like indicator-protein binding or media turbidity. In contrast, electrochemical sensors, though bulkier, are more robust and reliable in such scenarios [7].
Automation and Feedback Systems
Automation has revolutionised bioreactor control, cutting down on human error and improving consistency. Automated systems with closed-loop controls are particularly valuable for long-term cultivated meat production [8]. For instance, a 2022 study from Chiang Mai University introduced a 3D-printed automatic bioreactor with colourimetric pH monitoring. This system maintained a pH of 7.4 ± 0.2 and achieved over 80% cell viability, significantly enhancing cell proliferation over 72 hours compared to manual media changes [8].
Another notable example comes from Merck Biodevelopment in Martillac, France. In December 2013, the team tested the Mobius CellReady 3L single-use bioreactor for perfusion processes. Using Alternative Tangential Flow (ATF) technology for automated cell retention and media exchange, they achieved a 2.9-fold increase in monoclonal antibody production compared to batch mode. Researchers Aurore Polès-Lahille and Flavien Thuet reported that this automated system supported cell densities of 33 million cells/mL while maintaining pH levels between 6.80 and 7.10 [10]. These systems provide continuous data, enabling real-time adjustments to optimise cell growth and productivity [8].
Advanced bioreactors, sensors, and control systems for cultivated meat production are available through suppliers such as Cellbase.
sbb-itb-ffee270
Study Results: pH and Temperature Control Outcomes
Automated vs Manual Control Systems
In April 2022, researchers Suruk Udomsom, Pathinan Paengnakorn, and their team at Chiang Mai University tested an automated programmable bioreactor using L929 mouse fibroblast cells. This system carried out partial media refreshes every 6 hours over a 72-hour period. The results? Cell proliferation was significantly higher in the automated system compared to traditional manual culture methods. The bioreactor maintained a stable pH of 7.4 ± 0.2, with cell viability consistently above 80% throughout the experiment [8].
Manual systems, by contrast, face challenges. When media is removed from a CO₂ incubator for inspection, it starts to alkalinise almost immediately, with a time constant of 2–3 hours. Once placed back into the incubator, it takes roughly 45 minutes to return to the correct pH [2]. These fluctuations can destabilise cells. Automated systems, however, are designed to eliminate such inconsistencies, ensuring a more stable environment for cell growth.
Testing Different pH and Temperature Ranges
In April 2019, Johanna Michl and her team at the University of Oxford explored the metabolic activity of DLD1 cells over a 6-day incubation period. When glucose levels were kept above 12 mM, the cells produced approximately 20 mM of lactic acid, leading to medium acidification. The study found that even minor deviations from the optimal pH of 7.4 - specifically, shifts greater than 0.3 units - reduced proliferation rates across three mammalian cell lines: NCI-H747, DLD1, and Caco2 [2][3].
"Cellular growth... was optimal at pH 7.4, but when medium pH deviated from 7.4 by > 0.3 units all three cell lines exhibited reduced rates of proliferation." – Shannon G. Klein et al. [3]
In standard batch cultures, pH changes are common due to metabolic activity. High-density cultures, in particular, can experience dissolved oxygen levels dropping to as low as 0.95% [3]. These findings highlight how vital it is to maintain environmental stability, especially when scaling up production for cultivated meat.
Results for Cultivated Meat Cell Types
Expanding on controlled studies, scale-down simulations have shed light on the challenges of maintaining pH and temperature stability in large bioreactor systems. In July 2017, researchers at the Vienna University of Technology, led by Matthias Brunner and Jens Fricke, used a two-compartment scale-down model to mimic conditions in a 10–12 m³ stirred tank bioreactor. They exposed CHO cells to brief periods of pH 9.0 to simulate inhomogeneities caused by base addition in large-scale systems. Even short-term exposure to such elevated pH levels disrupted the specific growth rate during the exponential phase, resulting in reduced maximum viable cell density and lower product yield [4].
"Even short-term exposure of cells to elevated pH values during large-scale processes can affect cell physiology and overall process performance." – Matthias Brunner et al. [4]
In some mammalian cell cultures, around 90% of glucose is metabolised into lactate, which underscores the need for active pH buffering. These findings emphasise the critical role of precise environmental control throughout the production process to ensure optimal cell growth and productivity.
Equipment Selection and Bioreactor Scale-Up
Bioreactor Types Comparison for Cultivated Meat Production
Design Requirements for pH and Temperature Control
Bioreactors used for cultivated meat production need to have precise control systems to maintain a narrow pH range of 7.2–7.4 [1]. Advanced systems like nonlinear model predictive controllers (NMPC) and adaptive controllers are particularly effective at regulating feeding rates while keeping pH and temperature stable [12]. Automated feedback systems also play a key role in eliminating inconsistencies caused by manual adjustments.
For stable pH regulation, CO₂/bicarbonate buffering is highly effective. CO₂ acts as a self-buffering agent near neutral pH and is non-corrosive, making it a suitable choice [1][2][11]. To handle the metabolic heat produced during cell growth, bioreactors should be equipped with heat exchangers or service fluid flow systems [1][12].
Cultivated meat cells, particularly myocyte precursors, are highly sensitive to hydrodynamic stress due to their anchorage-dependent nature. These cells are far more fragile than suspension-adapted cells [1]. To protect them, bubble-free aeration methods like gas-permeable silicone tubing are preferred over traditional sparging techniques, which can cause damaging shear stress [1][11]. Additionally, integrating high-quality sensors - such as in-line probes for pH and dissolved oxygen (pO₂), along with off-gas sensors for monitoring carbon dioxide tension (pCO₂) - enables real-time environmental control [13].
Although these control strategies work well in smaller systems, maintaining the same level of precision becomes increasingly complex as the bioreactor size increases.
Scaling Challenges in Larger Bioreactors
Scaling up bioreactors from lab settings to commercial production introduces a host of challenges. At larger volumes, gradients in hydrogen ion concentration, carbon dioxide, and dissolved oxygen can emerge, leading to uneven environmental conditions [13][14]. These inconsistencies are particularly problematic for cultivated meat, where uniform cell growth is critical. For instance, in large-scale fed-batch processes, dissolved CO₂ (dCO₂) levels can reach 75–225 mg/L, while dissolved oxygen remains below 8.0 mg/L [11]. This accumulation of CO₂ can cause pH levels to drop as low as 6.8 [13].
"Understanding of process parameter interactions is especially useful during process scale-up, where unwanted variations of pH, dissolved oxygen tension (pO₂) and carbon dioxide tension (pCO₂) are most likely to occur." – Matthias Brunner et al. [13]
Maintaining a consistent temperature of 37°C is another critical factor, requiring continuous removal of metabolic heat [1]. Achieving this balance involves sufficient agitation to ensure homogeneity, but excessive impeller speeds can damage shear-sensitive cells [1]. To address these issues at commercial scales, it may be necessary to decouple pH and pCO₂ control. For instance, using HCl or NaOH for pH adjustments instead of relying solely on CO₂ gas can prevent CO₂ toxicity while maintaining stable pH levels [13].
| Bioreactor Type | Achievable Cell Density (cells/mL) | Working Volume for 1 kg Biomass |
|---|---|---|
| Stirred Tank (STR) | 1.90 × 10⁵ – 2 × 10⁶ | 570 L |
| Packed Bed (PBB) | 2.93 × 10⁶ | 110 L |
| Hollow Fibre (HFB) | 10⁸ – 10⁹ | 1.4 L |
Sourcing Equipment Through Cellbase
Finding bioreactors that meet the specific demands of cultivated meat production can be a daunting task. Cellbase simplifies this process by connecting researchers and production teams with trusted suppliers of specialised equipment. From stirred tank bioreactors with low-shear impellers to advanced sensor technologies for monitoring pH and pCO₂, Cellbase offers a curated selection tailored to the cultivated meat industry.
Unlike general lab supply platforms, Cellbase focuses solely on the needs of this sector. It provides detailed listings that include information such as shear-sensitivity ratings and buffer compatibility. Users can compare bioreactor types, request quotes directly from suppliers, and access insights on equipment trends. Whether you're sourcing single-use bioreactors up to 2,000 L [1] or exploring perfusion systems for high-density cultures, Cellbase equips you with the tools and knowledge to make informed decisions. By addressing the unique challenges of scaling up, the platform helps streamline the equipment selection process for both laboratory and commercial-scale production.
Conclusion
Maintaining precise pH and temperature control is absolutely crucial in cultivated meat production. These factors directly impact cell viability and growth consistency. Even a minor deviation - just 0.3 pH units outside the optimal range - can significantly hinder cell proliferation [3]. Similarly, stable temperature is essential for preserving the metabolic balance that supports cell growth. Johanna Michl from the University of Oxford highlights this sensitivity, noting:
"Biological processes are exquisitely sensitive to acid–base chemistry" [2]
This precision becomes even more challenging at commercial scales, where maintaining homeostasis across large volumes introduces significant engineering hurdles.
The shift from manual laboratory methods to automated bioprocesses is a key milestone for making cultivated meat production financially sustainable and reproducible. Automation eliminates the inconsistencies tied to manual monitoring. Advanced bioreactor systems - ranging from stirred tanks to hollow fibre setups - offer varying cell density capabilities while also affecting the facility's physical footprint and media efficiency.
However, scaling up brings its own set of complications. Large-scale bioreactors, often in the range of 10–12 m³, are particularly prone to pH inconsistencies. For instance, localised pH spikes can reach up to 9.0 during base additions [4], underscoring the need for robust control mechanisms. Shannon G. Klein from the Red Sea Research Centre stresses the importance of maintaining stable conditions:
"Maintaining relevant physiological conditions in cell cultures is of paramount importance to ensure the reproducibility of published findings and the translational relevance of experimental data to clinical applications" [3]
To tackle these challenges, specialised equipment and advanced monitoring systems are essential. Platforms like Cellbase provide valuable resources for sourcing the necessary technologies. These include low-shear impellers and integrated systems capable of real-time monitoring of pH, pCO₂, and dissolved oxygen, ensuring precise environmental control.
With over 175 companies now active in the cultivated meat industry across six continents and investments surpassing £2.4 billion [15], maintaining optimal pH and temperature conditions is pivotal for commercial success. Innovations in bioreactor design, automation, and specialised procurement are enabling the industry to transition from research labs to large-scale production facilities. These advancements are shaping the future of cultivated meat, helping the sector overcome its most pressing challenges.
FAQs
Why is it important to control pH and temperature during cultivated meat production?
Precise control over pH and temperature is absolutely critical when producing cultivated meat, as mammalian cells are highly sensitive to even minor environmental changes. Most cell lines used in this process thrive at an optimal temperature of around 37°C. However, even slight fluctuations - such as temperatures exceeding 38°C or dropping too low - can significantly impact cell viability, slow their growth, or interfere with metabolic functions. Similarly, keeping the pH stable within the range of 7.0 to 7.4 is equally important. Shifts in this range, often caused by metabolic by-products like CO₂ or lactate, can harm cell growth and compromise tissue quality.
In large-scale bioreactors, maintaining uniform pH and temperature throughout the entire system becomes even more critical. Consistent regulation across the bioreactor ensures predictable cell development and supports the growth, differentiation, and texture of the final product. It also helps minimise costly trial-and-error adjustments during production. For researchers and manufacturers in the cultivated meat industry, platforms such as Cellbase provide specialised tools tailored to meet these stringent requirements.
How do automated bioreactors enhance cell growth compared to manual systems?
Automated bioreactors bring a new level of precision to managing key parameters such as temperature, pH, and dissolved oxygen, ensuring the ideal conditions for cell growth. For example, these systems typically maintain temperatures around 37°C and pH levels between 7.0 and 7.4. Equipped with advanced sensors, they continuously monitor these variables and make rapid adjustments - whether it's heating, cooling, regulating gas flow, or balancing acid and base levels. This near-instant response eliminates the delays and inaccuracies often seen with manual interventions. The result? A stable environment that minimises cell stress, boosts metabolic efficiency, and promotes higher growth rates and cell densities.
On top of that, modern bioreactors utilise cloud-based analytics to track performance, optimise feeding schedules, and fine-tune processes across different production runs. In the context of cultivated meat, these innovations mean greater cell yields, accelerated tissue development, and lower production expenses. For those in the field, platforms like Cellbase provide access to the latest automated bioreactors and tools specifically designed for cultivated meat production.
What are the main challenges in scaling up bioreactors for cultivated meat production?
Scaling up bioreactors for producing cultivated meat is no small feat. As the size of these reactors grows, maintaining tight control over factors like pH, temperature, and dissolved gases becomes increasingly challenging. These fluctuations can lead to uneven cell growth and inconsistencies in the final product. Common hurdles include inefficient mixing, limited oxygen transfer, and slower sensor responses, all of which can disrupt the delicate balance needed for optimal cell culture.
Another layer of complexity comes from the use of adherent cell lines. These cells require either large surface areas or specialised micro-carrier systems to thrive. As systems scale up, it's crucial to support these cells properly without subjecting them to mechanical stress that could cause damage. On top of that, industrial-scale bioreactors must ensure uniform temperature distribution, maintain sterility, and adhere to strict food safety standards - all while keeping costs manageable.
To tackle these challenges, platforms like Cellbase provide access to cutting-edge tools. These include advanced bioreactors, high-precision sensors, and scalable micro-carrier technologies, equipping producers to meet the rigorous demands of large-scale cultivated meat production.











