ISO cleanroom classifications are essential for maintaining air cleanliness during cultivated meat production, ensuring product safety and minimising contamination risks. Here's a quick overview of how these standards apply:
- ISO 14644-1:2015 defines cleanroom classes based on airborne particle limits (0.1–5 µm), from ISO Class 1 (cleanest) to ISO Class 9 (least stringent).
- Cultivated meat production typically requires:
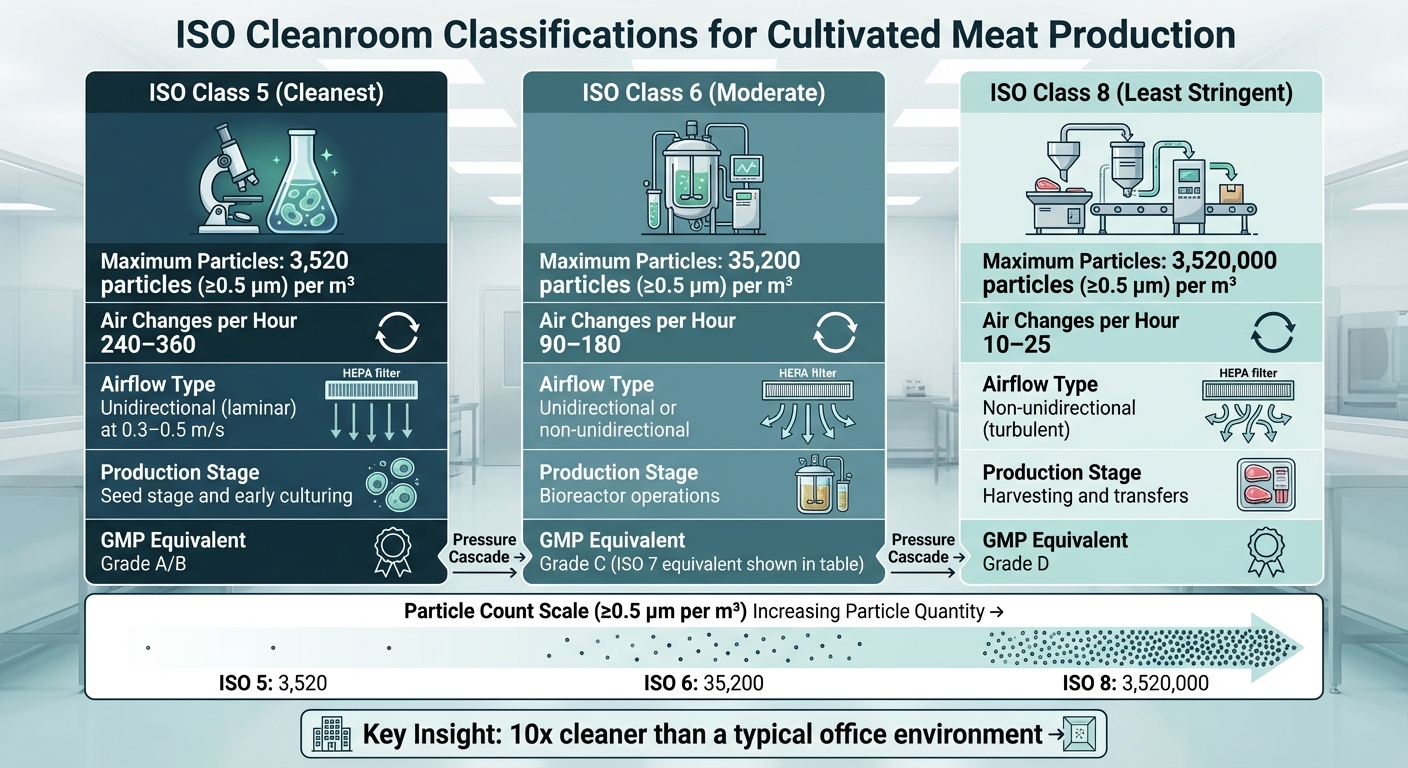
- ISO Class 5: For seed stage and early culturing, allowing up to 3,520 particles (≥0.5 µm) per cubic metre.
- ISO Class 6: For bioreactor operations, permitting up to 35,200 particles (≥0.5 µm).
- ISO Class 8: For harvesting and transfers, with a limit of 3,520,000 particles (≥0.5 µm).
- HEPA filters, airflow control, and pressure cascades maintain cleanliness. Higher air change rates (e.g., 240–360 per hour for ISO Class 5) are used in stricter environments.
- Cleanrooms are critical for cultivated meat as contamination can destroy batches, leading to financial losses and safety concerns.
The balance between cleanroom use and closed bioreactor systems can reduce costs while meeting safety standards. For example, closed systems minimise reliance on expensive ISO 5 environments, making production more cost-effective.
Key takeaway: Cleanroom classifications and proper environmental controls are vital for safe, efficient cultivated meat production, especially in high-risk stages like cell seeding.
ISO Cleanroom Classes for Cultivated Meat
ISO Cleanroom Classifications for Cultivated Meat Production Stages
Cultivated meat production relies on three main ISO cleanroom classifications - Class 5, Class 6, and Class 8. Each is designed to address specific contamination risks during different production stages, impacting both product safety and operational costs.
To maintain these standards, facilities often use a layered room design that creates pressure cascades to prevent contaminated air from entering critical areas [9]. Dr Heiko Baumgartner emphasises that "Classes 5 to 7 are mostly used in food production" [9], underscoring their importance in cultivated meat manufacturing. Below is a breakdown of how each ISO class applies to this process.
ISO Class 5: Seed Stage and Early Culturing
ISO Class 5 represents the cleanest environment in cultivated meat production, allowing no more than 3,520 particles (≥0.5 µm) per cubic metre [5][7]. At the seed stage, even the smallest contamination can jeopardise the entire batch.
To achieve this level of cleanliness, facilities use unidirectional (laminar) airflow at speeds of 0.3–0.5 m/s, combined with 240–360 air changes per hour [8][3][5]. These conditions align with EU GMP Grade A/B standards (at rest) [5]. The high rate of air changes ensures a continuous sweep of particles, maintaining sterility during critical operations like cell seeding and handling.
ISO Class 6: Bioreactor Operations
ISO Class 6 cleanrooms are less stringent than Class 5, permitting up to 35,200 particles (≥0.5 µm) per cubic metre. These zones operate with 90–180 air changes per hour, balancing strict control with practical usability [5][7][8][3]. Both unidirectional and non-unidirectional airflow methods can be employed [8][3].
GOOD Meat Inc. highlighted in their FDA consultation dossier that their cell expansion processes occur in cleanrooms equipped with HEPA filters and differential air pressure systems, adhering to biopharmaceutical standards [6]. This demonstrates how ISO Class 6 environments support large-scale cell growth while maintaining cleanliness.
ISO Class 8: Harvesting and Transfers
ISO Class 8 is the least restrictive classification used in cultivated meat production, allowing up to 3,520,000 particles (≥0.5 µm) per cubic metre [5][7]. Despite this higher threshold, it remains significantly cleaner than a typical office environment [7]. These areas are used for downstream processes such as harvesting, formulation, and post-harvest handling.
In November 2021, Mosa Meat noted that "the meat harvesting process … would likely be in an [International Standards Organisation] ISO Class 8 area" [6]. These zones require only 10–25 air changes per hour and rely on non-unidirectional (turbulent) airflow [8]. While more cost-efficient, they still provide adequate protection against environmental contaminants during final product handling.
| ISO Classification | Max Particles (≥0.5 µm/m³) | Air Changes per Hour | Typical Cultivated Meat Stage |
|---|---|---|---|
| ISO Class 5 | 3,520 | 240–360 | Seed stage and early culturing |
| ISO Class 6 | 35,200 | 90–180 | Bioreactor operations |
| ISO Class 8 | 3,520,000 | 10–25 | Harvesting and transfers |
Air Filtration and Environmental Control Requirements
ISO cleanroom standards require precise air filtration, controlled airflow, and stable environmental conditions to maintain particle levels within acceptable limits during cultivated meat production. These systems are carefully integrated into the facility's overall design to meet strict ISO classifications.
HEPA Filters for Air Quality
HEPA (High-Efficiency Particulate Air) filters are designed to trap particles as small as 0.3 µm [3]. In ISO Class 5 environments - commonly used for seed stage operations - HEPA filters often cover the entire ceiling, enabling unidirectional (laminar) airflow. This airflow moves downward at speeds between 0.3 m/s and 0.5 m/s, effectively sweeping particles out through floor-level exhausts [3].
In less stringent areas, like ISO Class 7 and 8 spaces, non-unidirectional (turbulent) airflow systems are typically used. These areas rely on higher air change rates to remove particles. For instance, ISO Class 5 rooms require 240–360 air changes per hour, while ISO Class 8 rooms only need 10–25 air changes per hour [3].
Air Changes, Pressure Cascades, and Monitoring
Air change rates are not one-size-fits-all. HVAC specialists calculate them based on factors like room size, the heat generated by equipment, and the number of personnel present, rather than applying generic standards [3]. Pressure cascades are another critical measure, ensuring cleaner zones maintain higher air pressure to push air toward less clean areas, reducing contamination risks. Airlocks and gowning rooms serve as physical barriers between zones with different ISO classifications [3].
To preserve pressure integrity, transitions between adjacent ISO classes must be carefully managed [3]. Real-time monitoring, as outlined in ISO 14644-2:2015, uses Light Scattering Airborne Particle Counters (LSAPC) to ensure particle concentrations stay within specified limits [1]. Additionally, the ISO 14644-1:2015 statistical model provides a 95% confidence level that at least 90% of the cleanroom area meets class limits [2].
Temperature and Humidity Control
Airflow management works hand in hand with maintaining stable temperature and humidity levels, which significantly impact particle behaviour and filtration performance. While ISO 14644-1 doesn’t prescribe specific temperature or humidity settings, these factors are critical for optimising filtration efficiency [2]. HVAC systems must account for heat generated by bioreactors and personnel to ensure consistent conditions [3].
Before conducting particle count tests, temperature and humidity should be stabilised to prevent interference with ISO classification results [2]. These environmental controls need to be incorporated during the facility's design and construction phases, as specified in ISO 14644-4, with tailored adjustments to meet the unique demands of cultivated meat production [4].
ISO Standards in Cultivated Meat Bioprocessing
ISO cleanroom classifications play a crucial role in cultivated meat production, aligning with each stage's needs to maintain cleanliness, prevent contamination, and ensure safety. These standards provide a framework for maintaining strict environmental controls throughout the process.
Proliferation and Growth Phases
During the proliferation phase, where cells multiply rapidly, maintaining a sterile environment is paramount. ISO Class 5 cleanrooms, equivalent to GMP Grade A/B in pharmaceutical manufacturing, are commonly used for seed stage operations and early cell culturing[11][13].
Compliance with these standards is essential. Dean Joel Powell highlights that cultivated meat produced in sterile conditions mirrors pharmaceutical standards, significantly reducing risks from pathogens like Salmonella, Campylobacter, and pathogenic E. coli[6].
Despite these measures, contamination remains a challenge. Industry reports show an 11.2% average contamination failure rate, which rises to 19.5% for larger-scale operations. In contrast, biopharmaceutical facilities - experienced with ISO protocols - reported only 3.2% contamination failures in 2022[6].
To balance sterility with cost efficiency, many facilities adopt a "room-in-room" design. This approach places an ISO 5 core within zones of lower cleanliness (ISO 6 or 7), using pressure cascades to direct airflow from cleaner areas to less critical zones, minimising cross-contamination[9]. For additional protection, facilities may use separative devices like clean air hoods or isolators, as specified in ISO 14644-7[4][12].
Once the proliferation phase ensures cellular integrity, the process transitions to harvesting, where ISO Class 8 environments take over.
Harvesting and Post-Harvest Handling
The harvesting phase, where cells are collected after maturation, operates in ISO Class 8 cleanrooms, equivalent to GMP Grade D[13]. At this stage, the cells are more stable and less prone to contamination compared to earlier growth phases. ISO 8 environments require fewer air changes - 10–25 per hour - compared to the 240–360 needed for ISO 5 spaces[8].
These cleanrooms reduce airborne particulates by a factor of ten compared to standard office environments, maintaining fewer than 3,520,000 particles (≥0.5 µm)[15]. This controlled environment ensures product quality during transfers and initial processing.
ISO 14698-1 provides guidelines for biocontamination control, including monitoring systems to detect bacteria and spores that could affect product safety[10]. Regulatory oversight also shifts during this phase. In the United States, the FDA supervises the proliferation and growth stages, while the USDA-FSIS oversees harvesting and subsequent processing[14].
Gowning and Workflow Protocols
Maintaining cleanroom standards also depends heavily on personnel protocols. ISO 14644-5:2025 outlines requirements for cleanroom operations, focusing on the movement of people and materials to preserve culture integrity[4].
Proper gowning is essential to prevent contamination from human sources like skin cells or microorganisms. Materials used for gowning must be compatible with the ISO class of the specific zone, as outlined in ISO 14644-18:2023[4]. Airlocks and gowning rooms act as barriers, ensuring contaminants are not carried between zones of differing cleanliness levels.
Training is another critical component. According to ISO 14698-1 Annex G, personnel must not only master gowning techniques but also understand the risks associated with contamination and the reasoning behind protocols[10]. This knowledge fosters vigilance, reducing procedural errors that could jeopardise entire production batches.
| ISO Class | GMP Grade Equivalent | Typical Bioprocessing Stage | Air Changes per Hour |
|---|---|---|---|
| ISO 5 | Grade A/B | Seed Stage, Early Culturing | 240–360 |
| ISO 7 | Grade C | Bioreactor Operations | 30–60 |
| ISO 8 | Grade D | Harvesting, Post-Harvest Handling | 10–25 |
| ISO 9 | N/A | General Facility/Support Zones | Variable |
sbb-itb-ffee270
Cleanrooms vs Closed Systems: Cost and Efficiency
When deciding on ISO classification for production, it's not just about meeting biosafety requirements. The choices you make also come with significant cost and efficiency implications that can influence commercial success.
Cleanroom Construction and Maintenance Costs
Building ISO-compliant cleanrooms is no small investment. Costs can range from £600 to £12,000 per m², depending on the classification level and technical specifications required [16]. To put this into perspective, constructing a cleanroom can be up to ten times more expensive than setting up an unclassified space for closed processing [17].
"It can be ten times more expensive - roughly $1,500 per square foot - to construct a clean room as opposed to an unclassified room." – Sebastian Bohn, Sub Market Leader, Alternative Proteins, CRB [17]
One of the biggest contributors to these costs is the HVAC system, which can account for 25%–50% of total expenses. For example, an ISO 6 cleanroom demands conditioning more than twice the air volume required for an ISO 8 environment [18]. And that’s not all - expenses like monitoring systems (ranging from £400 to £16,000+) and specialised features like interlocks or custom flooring are often left out of initial quotes [18].
Benefits of Closed Bioreactor Systems
Closed bioreactor systems offer a more cost-effective alternative to cleanrooms, while also improving biosafety. These systems allow cell cultivation to take place in sealed vessels, reducing the need for ISO-rated environments [17]. This approach not only cuts construction costs but also enhances safety through features like steam sterilisation and minimising vessel openings during sampling.
Dean Joel Powell from The Good Food Institute Asia Pacific has pointed out that classified cleanrooms may not be required for every step of production if the equipment is designed as a closed system. This is especially relevant for cultivated meat producers aiming to keep costs around £11 per kilogram, a stark contrast to the £40,000 per kilogram typical of biopharmaceutical production [6].
By relying on closed systems, producers can achieve a balance between affordability and safety, making it a practical choice for scaling up production.
Balancing Cost and Compliance
Taking a hybrid approach - combining closed systems with targeted cleanroom use - can help optimise costs while staying compliant with regulations. For instance, facilities might use closed systems for most bioprocessing steps, reserving cleanrooms for high-risk stages like seed culturing. This approach could reduce reliance on costly ISO 5 environments, which generally require 240–360 air changes per hour [8][19].
Different companies have taken varied approaches to this balance. GOOD Meat Inc., for example, uses cleanrooms with HEPA filters and differential air pressure, following biopharmaceutical standards for their entire process [6]. On the other hand, Mosa Meat has suggested that harvesting could occur in an ISO Class 8 area, the least stringent classification, while UPSIDE Foods has opted for "clean equipment" in temperature-controlled conditions for some operations [6].
Ultimately, producers must carefully weigh the trade-offs. Closed systems can substantially lower both capital and operational costs, all while potentially delivering better biosafety outcomes. This makes them an attractive option for many in the cultivated meat industry.
Conclusion
ISO cleanroom classifications play a critical role in managing contamination during cultivated meat production. For seed stage operations, maintaining an ISO Class 5 environment with 240–360 air changes per hour is typically necessary, while ISO Class 8 conditions are generally adequate for harvesting stages [8]. Although achieving pharmaceutical-grade sterility - completely removing pathogens - is technically possible, the associated costs are steep. For perspective, producing monoclonal antibodies costs around £40,000 per kilogram, whereas cultivated meat needs to reach approximately £11 per kilogram to remain commercially viable [6]. These financial constraints highlight the importance of adaptable contamination control strategies.
Industry leaders are already demonstrating how tailored cleanroom designs and closed system approaches can work within existing regulatory frameworks [6]. This balance between cleanroom standards and closed systems underscores the importance of ISO guidelines in cultivated meat production.
The key to success lies in strategic implementation. Facilities can combine closed systems for the majority of bioprocessing steps with selective cleanroom use for high-risk stages. This approach helps maintain biosafety while managing both capital and operational costs. As the industry progresses towards food-grade Good Cell Culture Practices (GCCP), such risk-based strategies will be increasingly crucial for meeting regulatory requirements and ensuring commercial viability [6]. For more detailed guidance on cleanroom optimisation in cultivated meat production, visit Cellbase.
FAQs
What are the advantages of using ISO Class 5 cleanrooms in cultivated meat production?
ISO Class 5 cleanrooms offer an environment with tightly regulated particle concentrations, ensuring an extremely clean and controlled space. This level of precision is essential for maintaining sterility and reducing contamination risks during critical aseptic processes in cultivated meat production.
Following ISO Class 5 standards helps facilities maintain product integrity, protect delicate cell cultures, and comply with strict biosafety and hygiene regulations. In cultivated meat production, even the smallest contamination can disrupt the entire process, making such controls indispensable.
How do closed bioreactor systems lower production costs in cultivated meat facilities?
Closed bioreactor systems play a key role in cutting production costs by drastically reducing the risk of contamination. This means less frequent cleaning and sterilisation, which saves both time and resources.
These systems also provide tightly controlled growth conditions, allowing for the efficient use of inputs such as growth media and energy. By boosting efficiency and limiting waste, closed bioreactors make producing cultivated meat more affordable and easier to scale.
Why is the air change rate crucial for maintaining cleanroom standards in cultivated meat production?
The air change rate plays a key role in upholding cleanroom standards in cultivated meat production. It ensures efficient control of airborne particles and microorganisms by frequently replacing the air within the cleanroom.
This process reduces contamination risks and helps maintain the necessary ISO cleanliness classification. Consistent air circulation not only safeguards biosafety but also protects product quality, providing the ideal conditions for cultivating meat cells while meeting strict industry requirements.











