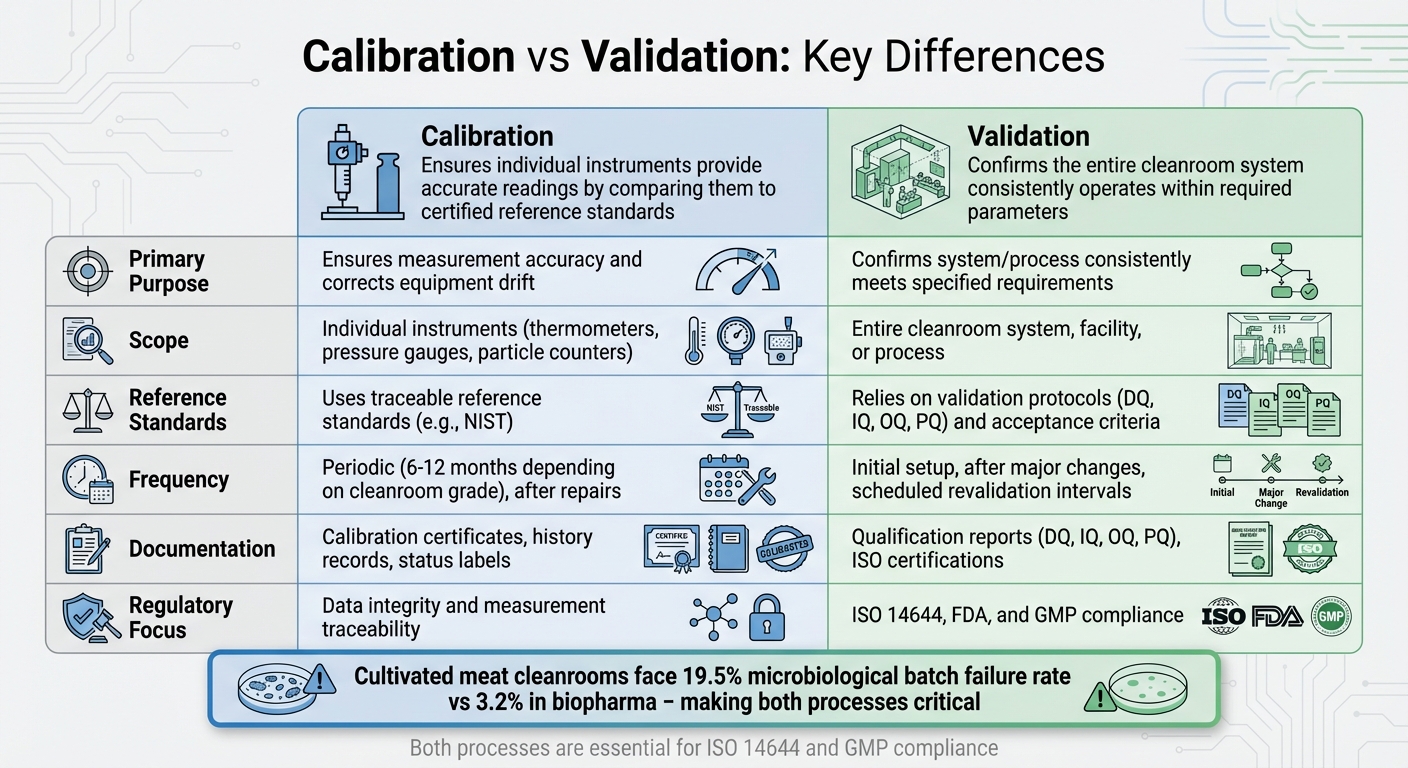
Calibration and validation are key to maintaining cleanroom standards for cultivated meat production. Here's a quick breakdown:
- Calibration ensures individual instruments like thermometers and pressure gauges provide accurate readings by comparing them to certified reference standards (e.g., NIST).
- Validation confirms that the entire cleanroom system, including equipment and processes, consistently operates within required parameters.
Why it matters:
- Cultivated meat cleanrooms face stricter demands compared to biopharma, with a microbiological batch failure rate of 19.5% (vs 3.2% in biopharma). Accurate calibration and thorough validation reduce these risks.
- Calibration focuses on individual devices, while validation assesses system-wide performance, ensuring sterility and regulatory compliance.
Key differences:
- Calibration is about accuracy for specific instruments.
- Validation evaluates overall system performance under real conditions.
- Both processes are critical for ISO 14644 and GMP compliance.
In short: Calibration ensures individual tools work correctly, while validation proves the system as a whole meets stringent cleanroom standards. Both are essential for reducing contamination risks and maintaining product quality in cultivated meat production.
Calibration vs Validation in Cleanroom Monitoring: Key Differences
What is Calibration?
Definition and Purpose
Calibration involves comparing the output of an instrument to a verified reference standard to eliminate any measurement bias [8]. As stated by NIST:
The purpose of calibration is to eliminate or reduce bias in the user's measurement system relative to the reference base [8].
In cultivated meat facilities, precision is non-negotiable. Accurate readings from temperature probes, particle counters, and pressure gauges are essential. Even small errors can disrupt sterility and lead to costly batch failures.
Key Processes in Calibration
Calibration typically follows a structured sequence of steps. First, technicians choose reference standards with known values that align with the equipment's operating range [12]. Before starting, they check the device for visible issues like contamination or sensor wear [11]. The next step involves comparing the instrument's readings to the reference values, generating a calibration curve [12]. If discrepancies arise, adjustments are made - either zero adjustments to fix constant offsets or span adjustments to correct the slope of the response curve [10]. Once the process is complete, a certificate is issued. This document includes measurement results, uncertainty values, and proof of traceability to national standards like those from NIST [11][2].
Calibration in Cleanroom Monitoring
In cleanroom settings, calibration is focused on instruments that measure critical parameters such as airborne particles, temperature, humidity, differential pressure, and airflow. For example, particle counters must comply with ISO 21501-4 standards and undergo calibration at least once a year [6][11]. Temperature sensors, whether thermocouples or Pt100 probes, also require routine calibration. Over time, factors like ageing and changes in metallurgy can cause these sensors to drift [10]. Similarly, differential pressure gauges and humidity probes need regular checks to ensure they meet the strict tolerances required for ISO 14644 compliance [6][2].
Antoine Nguyen, Director of Services at Dickson Data, explains:
Calibration strictly means the comparison of a measurement device to a known standard, which can be a material, object, physical process (like melting or freezing), or a second device which is known to be accurate [10].
It's essential to maintain records of both pre-adjustment ('as-found') and post-adjustment ('as-left') performance for audit purposes [10][7]. This meticulous process is a cornerstone of effective environmental monitoring. The next step is validation, which evaluates overall system performance.
What is Validation?
Definition and Purpose
Validation goes beyond individual instrument calibration to focus on the entire cleanroom facility. It's the formal process of ensuring that all systems, equipment, and monitoring tools work together to maintain the environmental conditions necessary for controlled manufacturing environments [5]. This isn't just about ticking regulatory boxes - validation is key to protecting product quality, ensuring process consistency, and safeguarding consumer safety. By confirming that critical factors like particle count, airflow, and pressure stay within set limits, validation keeps everything running smoothly. This is especially critical in cultivated meat bioprocessing, where sterility is non-negotiable. Validation ensures that cleanrooms perform reliably under real-world conditions, requiring a structured approach with specific qualification steps.
Key Processes in Validation
Validation follows a step-by-step sequence often referred to as the "Q series":
- Design Qualification (DQ): Ensures the cleanroom design aligns with required specifications and processes.
- Installation Qualification (IQ): Confirms that all components, such as sensors, HVAC systems, and monitoring devices, are installed correctly and according to approved designs.
- Operational Qualification (OQ): Tests key systems under static conditions to verify parameters like air velocity, pressure differentials, and particle counts perform as needed.
- Performance Qualification (PQ): Assesses the cleanroom's ability to maintain performance under real operating conditions, including normal personnel activity and equipment use.
Additional checks, like Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT), ensure equipment integrity during shipment. Every step is meticulously documented in a Validation Master Plan (VMP), with detailed test protocols and acceptance criteria, culminating in a final Validation Summary Report [5]. Together, these steps ensure the facility and its monitoring systems perform reliably.
Validation in Cleanroom Monitoring
In cultivated meat production, validation confirms that elements like HEPA filters, airflow patterns, and pressure differences effectively maintain sterility. While calibration ensures individual instruments are accurate, validation guarantees the entire system works as intended. For instance, HEPA filter integrity is tested using aerosol challenge methods to detect leaks. Smoke tests are used for airflow visualisation, ensuring unidirectional flow and identifying turbulence near sensitive areas. Pressure mapping verifies cascading pressure differentials to prevent cross-contamination between zones [5].
Environmental monitoring systems also undergo validation. According to ISO 14644-2, particle concentration must be tested every six months for ISO Class 5 facilities and annually for less stringent classifications [7]. Air velocity and pressure difference tests are typically conducted annually [7]. In ISO Class 7 cleanrooms - common in cultivated meat production - airflow must achieve 60 to 90 air changes per hour to meet standards [5]. Facilities generally schedule full or partial revalidation either annually, biannually, or following significant changes, such as new equipment installations, HVAC adjustments, or HEPA filter replacements [5].
Cleanroom Validation from URS to PQ
Key Differences Between Calibration and Validation
Calibration and validation are both essential for maintaining cleanroom standards, but they serve distinct purposes. Calibration ensures that individual instruments provide accurate measurements by comparing them to a traceable reference standard [13][2]. On the other hand, validation confirms that the entire cleanroom system consistently meets predefined acceptance criteria [13][15]. For instance, while calibration checks the accuracy of a thermometer, validation ensures that the cleanroom maintains the required environmental conditions.
The scope of these processes also varies significantly. Calibration targets specific hardware, such as sensors, particle counters, and pressure gauges, to identify and correct any measurement drift over time [13]. Validation, however, evaluates the cleanroom system as a whole, covering its design, installation, and operational performance [13][14]. This involves rigorous testing of parameters like HEPA filter efficiency, airflow patterns, and pressure differentials [13][14]. While calibration is typically performed more regularly on individual instruments, validation involves extensive system-wide assessments, making their roles complementary but distinct.
"Validation is the process that ensures that a system, product or services consistently provides results within the acceptable criteria." - SIC Web [13]
Another key difference lies in their role in regulatory compliance. Calibration depends on traceable reference standards to verify measurement accuracy [13][17]. Validation, on the other hand, follows protocols such as Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) to ensure compliance with standards like ISO 14644 [13][14][15]. Notably, calibration often precedes validation; all monitoring equipment must be calibrated before it can be used for Performance Qualification testing [15].
Summary of Key Differences: Calibration vs Validation
| Aspect | Calibration | Validation |
|---|---|---|
| Primary Purpose | Ensures measurement accuracy and corrects equipment drift [13][2] | Confirms a system or process consistently meets specified requirements [13][14] |
| Scope | Focuses on individual instruments (e.g., thermometers, pressure gauges) [13][17] | Evaluates the entire cleanroom system, facility, or process [13][15] |
| Reference Standards | Uses traceable reference standards for accuracy verification [13][17] | Relies on validation protocols and predefined acceptance criteria [13] |
| Frequency | Performed periodically, after repairs, or when results are in doubt [13][17] | Conducted during initial setup, after major changes, or at scheduled revalidation intervals [13][14][15] |
| Documentation | Includes calibration certificates, history records, and status labels [17] | Involves qualification reports (DQ, IQ, OQ, PQ) and ISO certifications [14][15] |
| Regulatory Focus | Ensures data integrity and measurement traceability [17] | Demonstrates compliance with ISO 14644, FDA, and GMP standards [14][15][16] |
When to Use Calibration
Routine Monitoring and Quality Assurance
Calibration plays a pivotal role in maintaining the integrity of cleanroom operations, especially during routine monitoring and quality assurance processes. It ensures that the data collected by sensors is accurate and dependable, which is crucial for overseeing daily operations effectively [1].
Every piece of monitoring equipment - whether it’s a temperature probe, humidity sensor, differential pressure gauge, or particle counter - needs regular calibration to ensure precise measurements. Even the slightest deviation in these readings can jeopardise product quality or lead to non-compliance with regulations.
The frequency of calibration depends on the cleanroom's classification. For cleanrooms classified under Grade A and B (ISO Class 5-6), calibration is generally required every 6 months, while Grade C and D (ISO Class 7-8) cleanrooms typically follow a 12-month calibration schedule [18]. Particle counters, in particular, must adhere to ISO 21501-4 standards, which require at least annual calibration [6]. To stay on top of these schedules, many facilities rely on calibration management software that sends automated reminders, ensuring deadlines are met and monitoring data remains valid [6]. Regular calibration not only keeps equipment performing accurately but also helps detect sensor drift early, reinforcing a strong quality assurance framework.
Detecting and Correcting Equipment Drift
Beyond routine schedules, calibration is instrumental in identifying and addressing sensor drift, which can occur due to ageing components or environmental factors [4].
When sensors drift from their original accuracy, calibration helps pinpoint the issue and apply adjustments at multiple calibration points. This process ensures the sensor’s output aligns correctly with the standard. To confirm the adjustment is effective, the calibration procedure is repeated, verifying that the device is now providing accurate readings [1].
"If a device is not calibrated correctly, it can lead to incorrect data and decision-making, which can have serious consequences, particularly when dealing with sensitive materials such as blood or medicine." - Fernanda Legarreta, XiltriX [1]
Continuous environmental monitoring systems can also reveal performance drift over time by analysing data trends. These insights allow for proactive maintenance, ensuring compliance isn’t compromised [5]. Additionally, certificates verifying NIST traceability are crucial for audit purposes, providing the necessary documentation to demonstrate calibration accuracy [1].
sbb-itb-ffee270
When to Use Validation
Initial Qualification and Installation
Validation plays a key role when setting up new equipment or commissioning a cleanroom. While calibration ensures accurate measurements, validation takes it a step further by confirming that the entire system aligns with design specifications and meets regulatory standards [2][19].
This process typically follows three stages: IQ (Installation Qualification) to verify proper installation, OQ (Operational Qualification) to ensure stable performance under different conditions, and PQ (Performance Qualification) to confirm that the system consistently meets required performance levels. These steps ensure that monitoring systems are reliable and ready for critical cleanroom control before being put into operation [20].
Changes in Cleanroom Processes or Equipment
Re-validation becomes necessary when significant changes occur that could impact cleanroom performance. This includes events like major HVAC failures, replacing terminal filters, correcting non-compliance issues, or relocating monitoring equipment. Each of these scenarios requires a new validation cycle to ensure that environmental conditions remain uncompromised [7][19].
As ISO 14644-2 states:
"The facility must be re-evaluated after... special maintenance that seriously affects the operation of the facility (i.e. changing the terminal filter)." - ISO 14644-2 [7]
These changes not only affect operations but also trigger regulatory requirements, making validation a critical step to maintain compliance.
Regulatory and Compliance Requirements
In GMP-compliant environments, validation isn't just good practice - it's a legal necessity. For facilities producing cultivated meat, for example, validation provides documented evidence that environmental conditions are consistently controlled to ensure product safety [20].
The updated EU GMP Annex 1, which came into effect on 22nd August 2022, introduces a Contamination Control Strategy (CCS). This strategy outlines all critical control points that require validation [6]. For facilities using continuous monitoring systems, compliance testing intervals can be extended as long as results consistently stay within acceptable limits [6][7]. Validation ensures that key parameters - such as temperature, humidity, differential pressure, and particle counts - are accurately measured, reducing the risk of contamination in sensitive bioprocessing environments [2][6].
Comparison of Processes and Testing Methods
Calibration and validation each play a specific role in ensuring cleanroom compliance, relying on distinct testing approaches to meet regulatory standards. These methods are especially critical in cultivated meat cleanrooms, where strict protocols must be followed. Calibration zeroes in on individual instruments, like particle counters, temperature sensors, and humidity probes, comparing their readings against certified reference standards to confirm accuracy [2][15]. On the other hand, validation takes a broader view, assessing the entire cleanroom system to confirm it meets ISO 14644-1 cleanliness classifications [7].
The regulatory requirements for these processes also differ. Calibration of particle counters must comply with ISO 21501-4 to ensure accurate counting and sizing of airborne particles [6][9]. Validation, however, adheres to ISO 14644-1 for cleanliness classification and ISO 14644-3 for specific procedures, such as airflow velocity measurements and pressure differential testing [7]. These regulations also dictate how frequently each process must occur.
For example, ISO 21501-4 mandates annual calibration of light-scattering particle counters [6][9]. Validation, however, often follows a more frequent schedule. Cleanrooms rated ISO Class 5 or cleaner require particle concentration tests every six months, while those above ISO Class 5 need testing annually [7]. Other tests, like air velocity, air volume, and pressure difference measurements, typically need validation every 12 months. Optional tests, such as filter leakage and airflow pattern assessments, are recommended every 24 months [7].
Calibration is also a prerequisite for reliable validation. According to ISO 14644-2, all instruments used during validation must be properly calibrated to ensure data accuracy and integrity [7]. Without this, validation results can become unreliable, potentially compromising compliance [3][2].
Comparison Table: Testing Methods and Applications
| Test Type | Calibration Application | Validation Application |
|---|---|---|
| Particle Count | Comparing particle counter readings against certified standards for accuracy [2][15] | Testing airborne particle concentrations under dynamic conditions to certify ISO classification [7] |
| Filter Integrity | Not applicable | Conducting aerosol challenge tests with photometers to verify HEPA filter efficiency of ≥99.99% [15] |
| Environmental Checks | Calibrating temperature and humidity sensors using calibration baths and generators [21] | Validating overall environmental conditions with calibrated data loggers for continuous monitoring [14][15] |
| Airflow Testing | Not typically a calibration parameter | Measuring air velocity with anemometers and visualising airflow patterns with smoke generators [21][14] |
| Pressure Differential | Calibrating pressure sensors with pressure calibrators [21] | Ensuring pressure differences between zones remain within 1–20 mmHg using wall-mounted manometers [14] |
Importance in Cultivated Meat Bioprocessing Cleanrooms
Ensuring Product Safety and Quality
Precision is the backbone of ensuring product safety in cultivated meat production. Calibration and validation work together to eliminate contamination risks, as even the slightest environmental variation can jeopardise entire batches. For instance, calibrated sensors that monitor temperature, dissolved oxygen (DO), pH, and glucose levels in bioreactors provide the crucial data needed for animal cell proliferation and maturation [22]. If these sensors lack accuracy, the risk of contamination or failed cell growth increases significantly [22].
Validation complements calibration by ensuring that every element of the cleanroom environment - such as HVAC systems, air filtration, and personnel protocols - functions as intended, even under challenging conditions like power outages or frequent door openings [1][5]. Performance Qualification (PQ) goes a step further by confirming that the cleanroom consistently meets safety and quality standards during actual production activities [5]. This meticulous approach is especially vital in cultivated meat production, which avoids the use of antibiotics and hormones commonly found in traditional livestock farming [22].
Supporting Regulatory Compliance
Regulatory bodies have set rigorous standards for cultivated meat production, demanding strict environmental controls. Compliance with frameworks like ISO 14644, EU GMP Annex 1, and FDA/USDA guidelines depends on detailed protocols including Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) [5][22].
A landmark example of regulatory success occurred on 2 December 2020, when the Singapore Food Agency (SFA) approved the commercial sale of cultivated chicken nuggets by Eat Just, a San Francisco-based company. This approval was granted after the company satisfied stringent safety assessments, which included thorough monitoring and validation of their production processes [22].
"Calibration is the process of comparing the measured value of a device or sensor to a calibrated standard in order to determine its accuracy" - Fernanda Legarreta, XiltriX [1]
Achieving compliance paves the way for sourcing the right equipment to maintain these high standards.
Sourcing Reliable Equipment via Cellbase
Meeting these demanding regulatory and operational standards requires access to specialised equipment. However, mainstream laboratory supply platforms often lack the expertise needed to cater to the specific needs of cultivated meat production. This is where Cellbase steps in. As the first specialised B2B marketplace dedicated to the cultivated meat industry, Cellbase connects researchers, production managers, and procurement specialists with reliable suppliers of essential cleanroom equipment.
The platform offers curated listings that include use-case specifications, such as GMP-compliance and bioreactor compatibility, helping teams identify equipment that meets ISO 14644 standards and other regulatory requirements. By focusing on the unique needs of cultivated meat production - like antibiotic-free environments and precise cellular microenvironment control - Cellbase simplifies the procurement process. This targeted approach ensures that teams have access to calibrated instruments and validation-ready systems from suppliers who understand the stringent cleanroom standards required for commercial-scale cultivated meat production.
Conclusion
Calibration and validation play a critical role in maintaining cleanroom standards. Calibration ensures that instruments like particle counters, temperature sensors, and humidity monitors deliver accurate and traceable data[2]. Meanwhile, validation confirms that the entire cleanroom facility and its systems operate in line with design specifications and regulatory requirements[5].
These processes are key to meeting international standards such as ISO 14644 and GMP. While calibration ensures the accuracy of collected data, validation demonstrates that the cleanroom environment is effectively controlled.
In the context of cultivated meat production, strict environmental control is non-negotiable. Calibrated sensors provide precise monitoring of temperature, humidity, and particle levels, ensuring a stable environment. At the same time, validation confirms that HVAC systems, filtration units, and personnel protocols perform reliably under all conditions. This thorough approach supports the demands of modern cleanroom management.
Advancing these practices, continuous environmental monitoring offers a forward-thinking alternative to traditional revalidation schedules. Real-time data enables predictive maintenance and ensures an audit-ready environment, allowing facilities to extend the time between formal cleanroom classifications. By adopting a risk-based approach, where revalidation is triggered by specific events rather than fixed dates, cleanroom management becomes more flexible and efficient[5].
Specialised solutions, like those provided by Cellbase, are tailored for cultivated meat companies. Their validated, GMP-compliant monitoring equipment helps maintain the stringent environmental controls required for regulatory compliance and commercial success. By supporting calibration and validation efforts, Cellbase ensures cleanroom integrity is upheld in this rapidly evolving industry.
FAQs
Why is validation more important than calibration in cultivated meat production?
Validation holds greater importance than calibration in cultivated meat production as it ensures that the entire system - spanning equipment, processes, and software - operates reliably and aligns with predefined standards. While calibration is about checking the accuracy of individual instruments against established benchmarks, validation takes a broader approach. It confirms that the entire process consistently achieves the desired results under actual operating conditions.
This distinction is crucial in cultivated meat manufacturing, where maintaining precise control over environmental factors and processes is directly tied to product quality, safety, and adherence to regulations. Validation guarantees that all components work harmoniously, safeguarding the quality of the final product and ensuring compliance with industry requirements.
How often should cleanroom monitoring equipment be calibrated and validated?
Cleanroom monitoring equipment needs regular calibration, guided by the manufacturer's recommendations, regulatory standards, and specific risk assessments. Depending on how critical the environment is and how the equipment is used, this process is typically scheduled quarterly, semi-annually, or annually.
Validation serves a different purpose - it’s carried out periodically to ensure the equipment consistently performs as required and complies with necessary standards. In industries like cultivated meat production, where precision is non-negotiable, both calibration and validation play a crucial role in upholding rigorous quality and safety requirements.
What happens if calibration and validation are overlooked in cleanrooms?
Neglecting calibration and validation in cleanrooms can cause significant problems, particularly in industries like pharmaceuticals, biotechnology, and cultivated meat production, where maintaining strict environmental controls is non-negotiable. If calibration isn't performed regularly, monitoring equipment might deliver incorrect readings for crucial factors like particle counts, temperature, humidity, and pressure. This could lead to undetected contamination, compromised product quality, and failure to meet regulatory requirements - potentially resulting in expensive product recalls or compliance violations.
Validation is just as important. It confirms that the entire monitoring system continues to operate as it should over time. Without validation, the reliability of data decreases, the risk of contamination increases, and the likelihood of regulatory breaches grows. For businesses producing sensitive items like cultivated meat, these lapses could mean losing entire batches, facing regulatory fines, and suffering damage to both reputation and customer confidence.
Consistent calibration and validation play a key role in preserving cleanroom standards, ensuring compliance, and safeguarding both product safety and operational performance.











