Scaling cell lines for cultivated meat production hinges on choosing the right bioreactor system. Costs vary significantly across stirred-tank, wave, and fixed-bed bioreactors due to differences in capital investment, operating expenses, and scalability. Here's what you need to know:
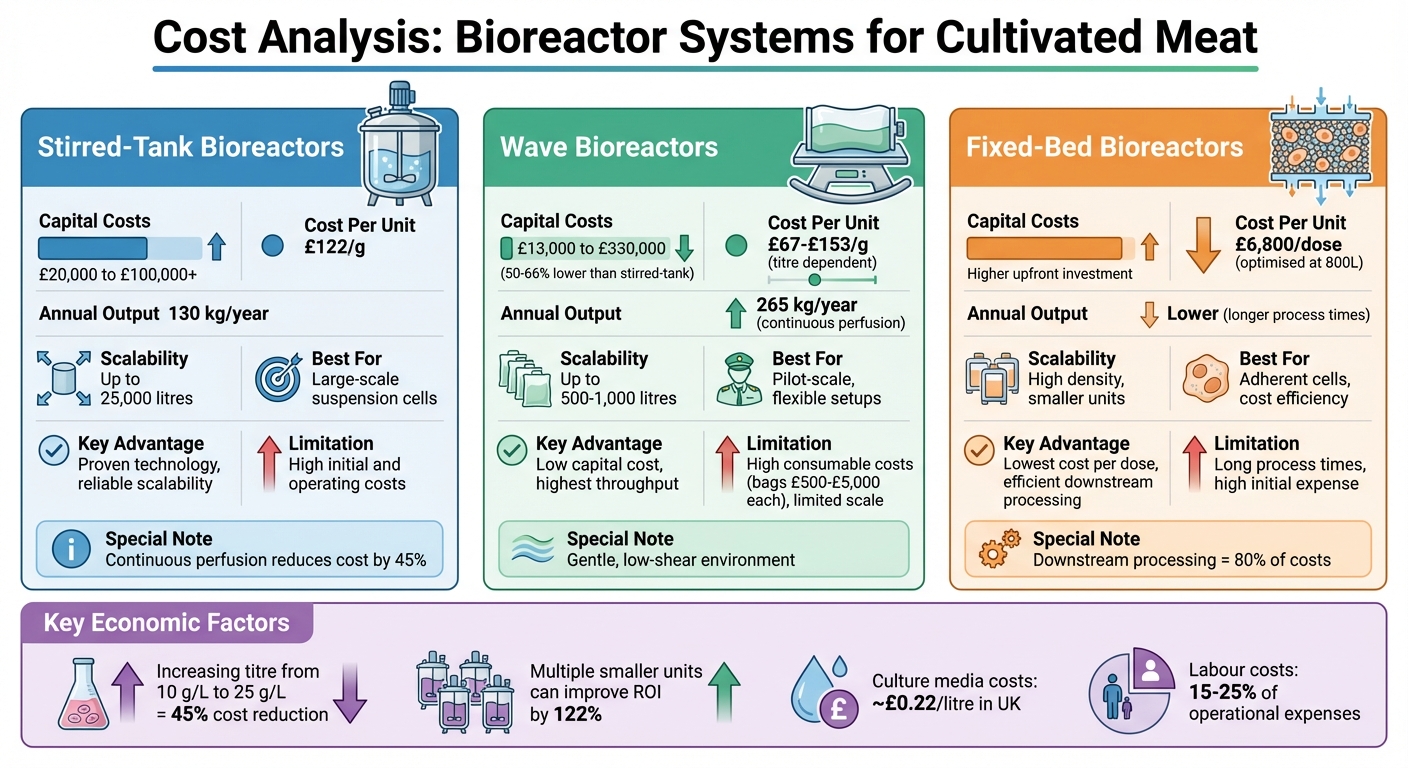
- Stirred-Tank Bioreactors: Best for large-scale production with suspension cell lines. High upfront costs (£20,000 to hundreds of thousands) but proven scalability (up to 25,000 litres). Continuous perfusion methods can reduce costs per gram by 45%.
- Wave Bioreactors: Affordable starting point (50–66% lower initial costs than stirred-tank systems). Ideal for small to medium scales but limited beyond 1,000 litres. Consumable costs (e.g., single-use bags at £500–£5,000 each) increase long-term expenses.
- Fixed-Bed Bioreactors: Suited for adherent cells, offering the lowest cost per dose at scale (£6,800 per dose at 800 litres). High initial investment but efficient for reducing downstream processing costs.
Quick Comparison
| Bioreactor Type | Capital Costs | Cost Per Unit | Scalability | Best For | Limitations |
|---|---|---|---|---|---|
| Stirred-Tank | £20,000+ | £122/g | Up to 25,000 litres | Large-scale suspension cells | High initial and operating costs |
| Wave | £13,000–£330,000 | £67–£153/g | Up to 1,000 litres | Pilot-scale, flexible setups | High consumable costs, limited scale |
| Fixed-Bed | Higher upfront costs | £6,800/dose | Smaller units, high density | Adherent cells, cost efficiency | Long process times, high initial expense |
Key Takeaway: Stirred-tank systems dominate large-scale production, while wave bioreactors are ideal for early-stage efforts. Fixed-bed systems excel in cost efficiency for adherent cell lines. The choice depends on production scale, cell line properties, and budget constraints.
Bioreactor Cost Comparison for Cultivated Meat Production: Capital, Operating Costs and Scalability
1. Stirred-Tank Bioreactors
Capital Costs
Investing in stirred-tank bioreactors is no small feat, with prices ranging from £20,000 for smaller bench units to several hundred thousand pounds for larger systems.[8] The choice of material plays a big role here. Stainless-steel systems, which are reusable, tend to cost 2–3 times more than single-use alternatives. This is mainly due to the added expense of steel vessels and the integrated Clean-in-Place (CIP) and Sterilise-in-Place (SIP) systems.[1] But the reactor itself isn’t the only major expense. Facility-related costs - like cleanrooms, HVAC systems, water-for-injection, and utilities - can make up more than half of the total project budget.[4] In the UK, meeting Food Standards Agency requirements for food-grade facilities adds yet another layer of cost. Tools like Cellbase can help producers compare supplier quotes and manage these expenses more effectively. Now, let’s dive into how operating costs influence the financial picture.
Operating Costs
Once the initial investment is made, the day-to-day running costs become a key factor. For stirred-tank systems, the biggest recurring expenses are growth media, consumables, and labour. In the UK, culture media costs are estimated at around £0.22 per litre.[6] Reusable systems offer a cost edge here, with operating expenses 20–40% lower than single-use formats, as there’s no need to keep buying disposable bags.[1] Stirred-tank systems also benefit from well-established protocols, which can reduce the amount of labour needed per batch compared to less automated setups. Process intensification, such as continuous perfusion techniques, can significantly cut costs. For example, studies show that continuous perfusion processes in stirred tanks can lower the cost per gram by about 45% compared to traditional fed-batch methods, thanks to increased productivity and reduced media usage per biomass unit.[4]
Scalability
When it comes to scalability, stirred-tank bioreactors are the gold standard. They’re available in sizes ranging from small bench-scale systems (1–5 litres) to industrial-scale units exceeding 10,000–25,000 litres.[4][7] A cost-modelling study found that at 1,000 litres, stirred-tank systems achieve a cost per dose of around US$12,000, making them more economical than multi-tray adherent systems.[3] Intensified processes further enhance scalability. For instance, continuous perfusion processes have been shown to nearly double annual product yields (265 kg compared to 130 kg) when compared to fed-batch processing, while also cutting capital costs by 32%.[4]
Cell Line Compatibility
Stirred-tank bioreactors excel with suspension-adapted cell lines that can handle hydrodynamic shear and thrive in well-mixed environments at high densities.[7] For cultivated meat production, this includes suspension-adapted myoblasts, satellite cells, or pluripotent stem cells grown in serum-free media. However, shear-sensitive cell lines require gentler mixing, which can limit oxygen transfer and cell densities, ultimately increasing media requirements and operating costs per kilogram of biomass.[7] Anchorage-dependent cell lines can also be cultured in stirred tanks using microcarriers, but this adds complexity and increases consumable costs, making them less cost-effective compared to fixed-bed systems. Cell lines with fast doubling times and high specific productivity can reduce reactor residence times and media usage, which economic models repeatedly highlight as key factors in lowering production costs.[4][7]
2. Wave Bioreactors
Capital Costs
Wave bioreactors present a more affordable starting point for cultivated meat producers, with upfront costs roughly 50–66% lower than those of reusable stirred-tank systems [1]. This cost advantage is largely due to their simpler mechanical design - there’s no need for complex impellers, drive motors, or integrated cleaning systems. In the UK, wave bioreactor units are priced between £13,000 and £330,000, depending on their size and level of automation [8]. Another key factor driving these savings is the use of single-use disposable bags, which eliminates the need for costly cleaning and sterilisation infrastructure. For startups or research teams working with tight budgets, this lower initial investment makes wave bioreactors an appealing choice for process development and pilot-scale production. Additionally, platforms like Cellbase allow producers to compare pricing from various suppliers, helping them find options that best align with their production needs. However, while these systems save on capital costs, they come with higher recurring consumable expenses, as explained below.
Operating Costs
When it comes to operating costs, wave bioreactors tell a different story. Consumable expenses, particularly the single-use bags priced between £500 and £5,000 each, contribute to higher long-term costs [5]. That said, wave systems do offer some operational benefits. Their gentle rocking motion uses less energy compared to the mechanical stirring of other systems, and they generally require fewer skilled staff for monitoring. However, the higher cost of consumables per batch means that long-term operating expenses tend to exceed those of reusable systems.
Scalability
Scalability is another area where wave bioreactors stand out - but with some limitations. They perform exceptionally well at small to medium scales but struggle beyond 500–1,000 litres, as the rocking motion becomes inefficient at larger volumes. This makes wave systems ideal for process development, pilot-scale production, and early-stage manufacturing, rather than large-scale commercial operations. A modular "scale-out" approach - using multiple smaller units in parallel rather than one large vessel - can improve return on investment by up to 122% compared to traditional single large-bioreactor strategies [2]. Additionally, since downstream processing typically accounts for around 80% of total production costs [2], sharing downstream equipment across multiple units can lead to further cost reductions. For cultivated meat production, this scalability profile supports a distributed manufacturing model, where multiple smaller facilities reduce construction costs and enhance supply chain resilience.
Cell Line Compatibility
Wave bioreactors are particularly well-suited for suspension-adapted cell lines and semi-adherent cultures. Their gentle, low-shear environment maintains high cell viability for cell types like immortalised muscle cells, fibroblasts, and pluripotent stem cells [3]. The choice of cell line can significantly impact production costs; for instance, increasing product titre from 10 grammes per litre to 25 grammes per litre can lower the cost of goods sold by about 45% [4]. The gentle mixing action of wave systems is especially advantageous for cell lines requiring longer culture periods, as it reduces cell damage and limits the need for frequent medium changes or expensive growth factor supplements. Although adherent cell lines can also be cultured in wave bioreactors using microcarrier beads, fixed-bed systems are generally a more economical option for these cell types.
3. Fixed-Bed Bioreactors
Capital Costs
Fixed-bed bioreactors require a substantial upfront investment due to the expense of specialised equipment and single-use vessels. A good example of this is the iCELLis® system, a well-known fixed-bed technology. At a clinical scale of 200 litres, the initial cost per dose was £17,000. This dropped to £8,500 per dose at 800 litres and further reduced to £6,800 per dose after optimising the production protocol [3]. While these capital costs may seem high, they become more manageable at larger production scales, thanks to the system's efficiency in processing throughput. For cultivated meat producers, platforms like Cellbase offer an avenue to compare costs and consult with verified suppliers of fixed-bed systems, helping them make informed decisions during procurement. Although the initial expenditure is steep, the long-term operational benefits often justify the investment.
Operating Costs
Despite their higher initial price tag, fixed-bed bioreactors deliver the lowest cost per dose when compared to other systems. For instance, at an 800-litre scale, the iCELLis® system produced doses at £8,500 each, significantly less than the £10,200 per dose for suspension bioreactors [3]. This cost advantage comes from better material utilisation and reduced downstream processing needs. In protein production, fixed-bed systems achieved a cost of £134 per gramme, while continuous fixed-bed processes brought this down to £100 per gramme [4]. However, costs are highly dependent on product titre. For example, when titre increased to 25 grammes per litre, costs dropped by around 45%. Conversely, a decrease to 10 grammes per litre pushed costs up to £156 per gramme [4]. Labour costs, which typically account for 15–25% of operational expenses in cultivated meat production, are also reduced due to the lower handling requirements of fixed-bed systems [1].
Scalability
Scalability is another area where fixed-bed systems shine, offering economic benefits through productivity gains rather than merely increasing vessel size. Though the iCELLis® system produces fewer doses annually compared to suspension bioreactors - owing to longer process times and immobilisation after seeding - it still emerges as the most cost-effective option when measured by cost per dose [3]. Its high surface area density allows for efficient large-scale cultivation without the need for enormous vessels. Additionally, using multiple smaller fixed-bed units that share downstream equipment can boost return on investment by 122% compared to using a single large bioreactor [2]. This scalability supports distributed manufacturing setups, which not only lower construction costs but also improve supply chain flexibility.
Cell Line Compatibility
Fixed-bed bioreactors are particularly well-suited for adherent cell lines that require a surface for growth. Their packed-bed design creates a high-density environment ideal for mammalian cells, including primary cells and stem cell lines, which are widely used in cultivated meat production [3]. The low-shear environment within the bed matrix protects cells from mechanical damage, making these systems an excellent choice for shear-sensitive cell types. Adherent cells with longer doubling times and specific microenvironmental needs benefit from the system's ability to precisely control nutrient gradients and waste removal through perfusion. Rapidly dividing cells, on the other hand, thrive in the immobilised setup, which ensures efficient nutrient delivery without the turbulence typical of stirred systems. However, selecting the right cell line is crucial, as even small gains in cell density or protein output per unit volume can lead to significant cost savings in fixed-bed operations.
sbb-itb-ffee270
Cost drivers of cultivated meat production
Advantages and Disadvantages
Choosing the right bioreactor system involves balancing initial investment, operational efficiency, and production costs. Here's a closer look at the strengths and weaknesses of different systems to help guide decision-making.
Stirred-tank bioreactors are a well-established option with proven scalability, making them a reliable choice for many industries. However, they come with the highest upfront cost (£41.2M) and the steepest cost per gramme (£122) [4]. While their control parameters are well understood, they require longer seed fermentation trains and have a lower annual production capacity (130 kg per year) [4].
Fixed-bed bioreactors stand out for their cost efficiency per dose, with an optimised cost of around £6,800 [3]. They excel in downstream processing, a critical factor since downstream costs can make up roughly 80% of total production expenses for high-value products [2]. On the downside, their longer processing times limit the number of batches produced annually [3].
Wave bioreactors and continuous perfusion systems strike a balance with a lower capital requirement (£28M) and the lowest cost per gramme (£67/g), while achieving the highest throughput (265 kg/year) [4]. However, their operational complexity and sensitivity to product titre can pose challenges. For instance, a drop in titre from 25 g/L to 10 g/L can increase costs to roughly £153/g [4].
The choice of bioreactor ultimately depends on factors like production scale, the properties of the cell line, and the achievable titre. Cellbase connects users with verified suppliers for all bioreactor types, ensuring tailored solutions for specific production and budgetary needs.
Here’s a quick comparison of the key metrics:
| Bioreactor Type | Capital Expenditure | Cost per Unit | Annual Throughput | Primary Advantage | Main Limitation |
|---|---|---|---|---|---|
| Stirred-Tank | £41.2M | £122/g | 130 kg/year | Reliable and scalable with proven technology | High capital and operational costs |
| Fixed-Bed | Higher CAPEX | ~£8,000/dose (optimised) | Lower (due to longer process) | Efficient downstream processing, low dose cost | Long process time, high initial investment |
| Continuous Perfusion | £28M | £67/g | 265 kg/year | Low cost per gramme, highest throughput | Complex to operate, sensitive to titre changes |
Conclusion
The cost-effectiveness of bioreactors depends heavily on the scale of production. For large-scale commercial manufacturing, continuous perfusion stirred-tank systems stand out, offering production costs of approximately £68 per gram compared to £124 per gram in fed-batch systems, with an impressive annual output of 265 kg [4].
For early-stage R&D and pilot-scale facilities, wave bioreactors offer a practical solution. Their lower upfront costs and quick setup make them ideal for start-ups in the UK working with limited budgets. Similarly, optimised fixed-bed systems can drive down costs per unit by supporting high cell densities and streamlining downstream processing [3]. These approaches allow smaller companies to minimise financial risks while perfecting their cell lines and processes.
When scaling out, using multiple smaller bioreactors can significantly improve returns. For example, ROI increases by 122% when downstream costs make up as much as 80% of total production expenses [2]. This strategy also helps reduce capital expenditure and the overall facility footprint.
Across all systems, advancements like higher cell densities, improved titres, and shorter process times play a critical role in cutting costs. For instance, increasing the titre from 10 g/L to 25 g/L can effectively halve production costs [4]. These economic considerations are key for producers aiming to choose the most suitable system for their needs.
FAQs
What should I consider when selecting a bioreactor for cultivated meat production?
When choosing a bioreactor for cultivated meat production, there are several key factors to consider. These include the specific needs of your cell line, the intended production scale, and the associated costs. Each type of bioreactor offers varying levels of efficiency, scalability, and compatibility, so it’s essential to match the equipment to your project's unique requirements.
Equally important is sourcing dependable equipment. Cellbase offers a specialised marketplace designed for the cultivated meat sector. Here, you can find bioreactors and other vital tools tailored to industry needs, simplifying the procurement process and ensuring access to reliable, high-quality solutions.
What are the differences in operating costs between stirred-tank, wave, and fixed-bed bioreactors?
Operating costs differ greatly among stirred-tank, wave, and fixed-bed bioreactors due to variations in their design, scalability, and how they use resources. Stirred-tank bioreactors are commonly used and are typically economical for large-scale production. However, they often require higher energy consumption for mixing and maintaining temperature. Wave bioreactors, in contrast, are easier to operate and tend to use less energy, making them a good choice for smaller-scale setups or early-stage development. Fixed-bed bioreactors, while having higher upfront costs due to specialised materials, can provide efficient resource usage and lower maintenance over time.
When setting up cultivation processes, it’s crucial to weigh these cost considerations against the unique requirements of your cell line and production objectives. Tools like Cellbase can assist in finding bioreactor systems and materials tailored to the cultivated meat sector, helping you achieve scalable and cost-efficient solutions for your projects.
What are the scalability challenges of wave bioreactors compared to other systems?
Wave bioreactors are popular for their straightforward design and affordability, especially in smaller-scale operations. That said, they can encounter hurdles when scaling up. As the volume increases, issues like reduced mixing efficiency and limited oxygen transfer can arise. These challenges may affect cell growth and overall productivity when transitioning to larger bioreactor systems.
In the case of cultivated meat production, selecting the ideal bioreactor system is all about finding the right balance between scalability, cost, and the unique needs of your cell lines. A thorough evaluation of these elements is crucial to achieving reliable performance at larger production scales.











