Maintaining precise pH levels is critical for cultivated meat production. Mammalian cells thrive in a narrow pH range (7.1–7.4), but metabolic acidification, CO₂ build-up, and mixing challenges make pH control complex, especially in large-scale bioreactors. Effective strategies include:
- Gas sparging: Removes excess CO₂ without raising osmolality or causing localised pH spikes.
- Advanced sensors: Potentiometric sensors offer high accuracy for stainless steel systems, while optical sensors work well with single-use bioreactors.
- Buffer optimisation: Adding buffers like HEPES improves stability but requires careful balance to avoid excess lactate production.
- Automated systems: Real-time adjustments using feedback loops ensure consistent pH levels.
These approaches help overcome challenges like lactic acid accumulation and shear stress, improving cell health and product yields.
Understanding pH Measurements in Bioprocess
Key Challenges in pH Management
This section delves into the main factors that contribute to pH instability, building on previously discussed challenges.
Metabolic Acidification and Lactic Acid Accumulation
Lactic acid is a major hurdle in cultivated meat bioprocessing. As cells metabolise glucose through glycolysis, they produce lactate and hydrogen ions in a 1:1 ratio. This process creates a significant acid load, making lactate the primary driver of medium acidification [1].
The buffering capacity of standard culture media - typically between 1.1 and 1.6 mM per pH unit [1] - is often insufficient during periods of rapid cell growth. As cells multiply, their metabolic waste output increases, overwhelming the medium's ability to maintain a stable pH. The sharp drop in pH during this phase can be directly attributed to glycolytic lactic acid production [1], underscoring lactate's pivotal role in destabilising the medium's pH.
The complications don’t stop there. CO2 accumulation adds another layer of complexity.
CO2 Build-Up and pH Drift
Cellular respiration introduces CO2 into the medium, where it dissolves to form carbonic acid. The key issue is the partial pressure of dissolved CO2 (pCO2), which influences whether CO2 can escape from cells. When pCO2 levels in the medium rise too high, CO2 becomes trapped inside cells, causing a dangerous drop in intracellular pH and eventually leading to cell death [2].
"If pCO2 is too high, CO2 can't leave the cells, so the intracellular pH will drop and the cells will die." - Alicat Scientific [2]
This problem becomes more pronounced in large-scale bioreactors. These systems have a lower surface-area-to-volume ratio, which reduces the efficiency of CO2 degassing compared to smaller vessels [3]. Even routine operations, like transferring media to a CO2 incubator, can cause pH fluctuations. For instance, small media volumes begin to alkalinise almost immediately, with a time constant of 2–3 hours [1].
Alongside chemical challenges, physical processes also play a significant role in pH instability.
Mixing and Shear Stress Impacts on pH Stability
Adjusting pH by adding a base introduces its own risks. When sodium bicarbonate or similar bases are pumped into bioreactors, poor mixing can create localised zones of high pH that harm nearby cells [2] [3]. On the other hand, the vigorous agitation needed to distribute the base uniformly can lead to shear stress and foam formation, both of which are damaging to fragile mammalian cells [2] [3].
In controlled experiments, base addition to stabilise pH often reduced cell viability due to increased osmolality [3]. This creates a difficult balancing act: insufficient mixing results in pH hotspots, while excessive mixing prevents hotspots but increases mechanical stress. The problem becomes even more challenging during scale-up, where longer mixing times make it harder to maintain effective pH control without compromising cell health.
Technologies for pH Monitoring and Control
Maintaining the pH within the narrow range of 7.1–7.4 is critical for mammalian cell cultures, demanding precise and reliable monitoring tools [2]. Potentiometric sensors, which act as electrodes to measure free hydrogen ions, are the gold standard for continuous pH monitoring in bioreactors [1]. These sensors provide real-time data, enabling automated systems to make immediate adjustments to maintain the required pH levels. Their high accuracy makes them essential for large-scale operations. Alongside these, optical indicators offer another effective way to measure pH.
Optical indicators rely on spectroscopic analysis to provide quantitative pH measurements. While phenol red is often used as a visual indicator, more precise readings are achieved through ratiometric analysis of absorbance at two specific wavelengths - 560 nm and 430 nm [1]. This method compensates for factors like media volume or dye concentration, ensuring consistent and accurate results.
"The concentration of free H+ ions is not intuitive to predict, but fortuitously simple to measure (e.g. with electrodes or indicator dyes)." - Johanna Michl et al., University of Oxford [1]
Modern pH control systems go beyond monitoring by integrating these measurements into automated feedback loops that dynamically regulate pH levels.
Automated feedback systems leverage sensor data to make real-time adjustments, eliminating the need for manual intervention. These systems can adjust pH by adding a base or using gas sparging techniques [2]. For large-scale bioreactors, gas sparging is particularly effective. Using mass flow controllers, CO2 levels can be adjusted quickly and evenly, ensuring uniform pH regulation [2]. In contrast, base pumping, while effective for smaller systems, can create localised pH imbalances and increase osmolality, making it less practical for larger vessels [2]. However, gas sparging requires careful attention to the design of spargers to avoid shear stress that could harm the cells [2]. For those in cultivated meat production, investing in advanced gas control systems can lead to better cell health and higher yields, making it a worthwhile expenditure.
sbb-itb-ffee270
Strategies for pH Management at Scale
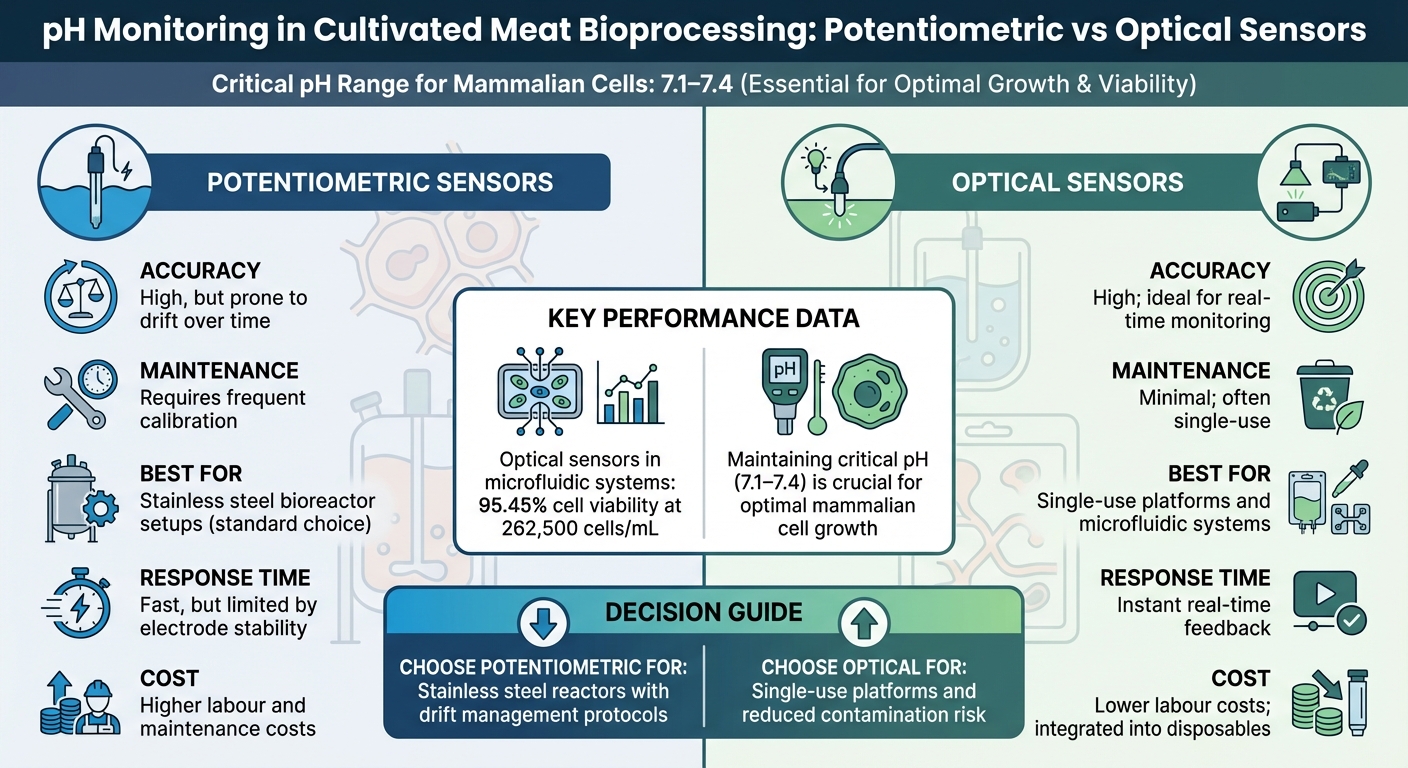
Potentiometric vs Optical pH Sensors for Cultivated Meat Bioreactors
Potentiometric vs Optical Sensors: A Comparison
Selecting the right sensor technology becomes increasingly important as cultivated meat production scales up. Potentiometric sensors are the go-to choice for stainless steel bioreactors due to their precision and quick response. However, they come with challenges like the need for regular calibration and susceptibility to drift during prolonged processes. Jacob Crowe, Applications & Tech Support Manager at Hamilton Company, explains:
"Over time, pH measurements can drift, which will impact the process's stability and performance. It's vital to monitor and mitigate pH drift to prevent detrimental effects on both metabolism and the overall process" [8].
On the other hand, optical sensors emerge as a practical option, particularly for single-use bioreactor systems. These sensors can be pre-installed in disposable bags, cutting down contamination risks and eliminating the need for sterilisation between cycles [7]. In microfluidic systems, optical sensors have shown excellent results, achieving cell viabilities of 95.45% at densities of 262,500 cells/mL [9].
| Feature | Potentiometric Sensors | Optical Sensors |
|---|---|---|
| Accuracy | High, but prone to drift | High; ideal for real-time monitoring |
| Maintenance | Requires frequent calibration | Minimal; often single-use |
| Scalability | Standard for stainless steel setups | Great for single-use and microfluidics |
| Response Time | Fast, limited by electrode stability | Instant real-time feedback |
| Cost Implications | Higher labour and maintenance costs | Lower labour; integrated into disposables |
The choice of sensor largely depends on the reactor type. Stainless steel bioreactors may benefit from potentiometric sensors with measures to manage drift, while single-use platforms can capitalise on the ease of integrated optical sensors [7] [8]. These decisions directly affect how pH stability is maintained during medium optimisation.
Medium Optimisation and Buffer Improvements
Once the appropriate sensors are in place, stabilising the culture medium’s buffering system becomes essential to maintain pH control during scale-up. Mammalian cells depend on the CO₂/HCO₃⁻ buffer system (pKa 6.15 at 37°C), but its buffering capacity is often insufficient. For example, standard DMEM with 10% FBS typically provides only 1.1 to 1.6 mM of buffering [1].
To address this, adding non-volatile buffers (NVBs) like HEPES (pKa 7.3 at 37°C) can significantly strengthen buffering without causing problematic osmolality shifts [1]. The recommended method involves titrating the medium to the target pH first, then adding NaHCO₃ at a concentration aligned with the incubator’s pCO₂. This approach reduces the initial pH drift when fresh media is exposed to CO₂, a process that can take up to two hours with NVBs [1].
However, stronger buffering systems may trigger increased glycolysis, leading to higher lactate production. In some cell lines, up to 90% of glucose is directly converted into lactate [1], and improved buffering can sometimes amplify this effect, resulting in greater lactic acid accumulation [10].
Sparging and Agitation Techniques
Gas sparging offers a practical way to manage pH in large-scale cultivated meat production. Alicat Scientific notes:
"Gas bubbles from spargers can be evenly mixed and distributed more quickly than base, and with much less agitation" [2].
By distributing gas bubbles uniformly, sparging provides a more consistent approach than chemical base additions. For instance, a 2018 study showed that maintaining constant sparge rates while increasing headspace aeration allowed titres to remain stable during scale-up from 30 L to 250 L [2].
Macro spargers, which produce bubbles 1–4 mm in diameter, are particularly effective at removing excess CO₂ from the culture. This raises pH naturally, avoiding the need for chemical bases that could elevate osmolality [2] [5]. A newer "gas-only" pH control strategy uses automated air sparging feedback loops. When pH drops, air flow increases to remove more CO₂. This method has been successfully scaled from ambr®250 bioreactors to 200 L vessels, maintaining precise pH levels throughout fed-batch cultures [6].
Balancing efficient gas transfer with minimal shear stress remains a critical challenge during scale-up. Airlift bioreactors, which use gas-driven circulation, offer a gentler mixing option with reduced shear stress. Computational fluid dynamics (CFD) simulations can also help identify high-shear zones near impeller blades, allowing bioreactor designs to be optimised before scaling up [4]. Combining these approaches with advanced tools from Cellbase can streamline pH management during scale-up.
Sourcing pH Control Equipment via Cellbase
Why Choose Cellbase for Procurement?
Precise pH control is essential in cultivated meat bioprocessing, making it crucial to source the right equipment. General lab supply platforms often lack the specialised knowledge required for the tight pH ranges in this field. Cellbase bridges this gap by connecting professionals with verified suppliers who meet these demanding standards [2].
By using Cellbase, procurement becomes more straightforward. The platform provides transparent pricing and industry-specific expertise, creating a curated marketplace for pH control technologies. Instead of juggling multiple suppliers across various channels, R&D teams and production managers can find everything they need in one place. This not only reduces the hassle of procurement but also minimises technical risks with its verified listings.
Finding pH Control Technologies Through Cellbase
Cellbase offers a wide range of pH management solutions, including potentiometric sensors, optical indicators, and automated feedback systems. These are compatible with both stainless steel and single-use bioreactors, catering to diverse operational needs.
For scaling up, the platform provides access to mass flow controllers and specialised spargers, which are critical for efficient gas-based pH management. As Alicat Scientific highlights:
"Keeping pH at healthy biological levels is potentially the most powerful tool in upstream bioprocessing to increase product titers" [2].
Additionally, Cellbase grants access to advanced Intelligent Sensor Management (ISM) technology. This system monitors sensor life, enabling predictive maintenance during extended batch processes [11].
Procurement specialists can also source equipment for CO₂ stripping, including autoclavable CO₂ sensors and single-use pH probes. These tools support scalable strategies for maintaining precise pH control, making it easier to integrate advanced pH management into large-scale production [11]. By offering targeted solutions, Cellbase simplifies the adoption of sophisticated pH control technologies across the production pipeline.
Conclusion: Best Practices for pH Control in Cultivated Meat Bioprocessing
Maintaining a pH range of 7.1 to 7.4 is critical for the survival of mammalian cells in cultivated meat production [2]. Keeping pH within this range plays a key role in improving product yields during upstream bioprocessing.
To address the challenges of pH control, several effective practices have emerged. One standout method is using gas sparging instead of base addition during scale-up. Gas sparging effectively removes excess CO₂ by distributing it evenly with minimal agitation, which helps avoid problems like pH inconsistencies and osmolality fluctuations [2]. A 2021 study by Aryogen Pharmed demonstrated the success of this method at a 250-litre scale, achieving a 51% increase in the final product yield [3].
Another important practice is direct pH monitoring, which provides a more comprehensive understanding of culture health compared to relying solely on pCO₂ measurements. This is particularly vital because dissolved CO₂ levels do not account for lactic acid build-up, which can make up as much as 90% of glucose metabolism in certain cell lines [1]. Monitoring pH directly becomes even more crucial during the exponential growth phase when metabolic activity peaks.
For non-volatile buffers like HEPES, it’s essential to consider buffer equilibrium. HEPES buffers can take up to two hours to stabilise and must be carefully titrated with bicarbonate and CO₂ [1]. However, increasing the buffering capacity may inadvertently boost lactate production, which can counteract the intended stabilising effect [1]. When combined with sensor-based monitoring and gas sparging techniques, these buffer considerations help maintain stable and optimal process conditions.
FAQs
How does gas sparging support pH control in cultivated meat production?
Gas sparging plays an important role in keeping the pH levels balanced during the production of cultivated meat. As cells grow, they release carbon dioxide (CO₂) as a by-product of respiration. This CO₂ can lower the pH of the culture medium, which may harm cell health. By introducing gases such as air, oxygen, or inert gases into the bioreactor, sparging helps remove excess CO₂. This prevents the medium from becoming too acidic and keeps the pH stable.
Maintaining the culture medium within the ideal pH range of about 7.1 to 7.4 is crucial for healthy cell growth and productivity. When paired with buffering systems and real-time monitoring using pH sensors, gas sparging not only improves process efficiency but also boosts cell viability. It’s a critical component in ensuring the success of cultivated meat bioprocessing.
What makes potentiometric sensors a better choice than optical sensors for pH monitoring in cultivated meat production?
Potentiometric sensors play an important role in cultivated meat production thanks to their ability to provide real-time pH measurements with high accuracy. Maintaining proper pH levels is essential for creating the right environment for cell growth, and these sensors excel in delivering the data needed to achieve that. On top of that, they're relatively affordable and integrate seamlessly into large-scale bioreactors, making them ideal for continuous monitoring in industrial settings.
What’s more, these sensors are built to handle the challenges of complex culture media, offering reliable performance even in demanding conditions. However, they do require periodic calibration to maintain their accuracy. With their blend of precision, reliability, and cost efficiency, potentiometric sensors have become a go-to choice for effective pH control in cultivated meat bioprocessing.
Why does lactic acid build-up make it difficult to maintain stable pH levels?
Lactic acid build-up complicates pH control by increasing the acidity of the culture environment, causing the pH to drop. This can harm cell viability and productivity, as most cells need a carefully controlled pH range to grow and function properly.
Managing lactic acid levels is crucial in cultivated meat bioprocessing to support healthy cell growth and maintain product quality. Approaches like real-time pH monitoring, the use of pH buffers, or adjusting feeding protocols can help stabilise the environment and avoid damaging pH swings.











