Validating growth media is a mandatory step for cultivated meat companies seeking approval in the UK market. This process ensures the safety, quality, and compliance of products under strict regulatory frameworks like the UK Novel Food Regulations (EU 2015/2283). Here's what you need to know:
- Key Requirements: Growth media must meet standards for toxicology, contamination control, nutritional quality, and allergenicity.
- UK Regulations: The Food Standards Agency (FSA) requires compliance with HACCP principles and classification under Products of Animal Origin (POAO).
- Global Standards: While the UK and EU share similar frameworks, the US follows CGMP regulations under the FD&C Act.
- Validation Process: Includes thorough testing of composition, purity, functionality, and supplier compliance, alongside robust documentation.
- Support Initiatives: The UK's £1.6 million regulatory sandbox, launched in 2025, aids companies in meeting these standards.
Proper validation ensures safety, builds trust, and aligns with legal requirements. The article dives deeper into the step-by-step process, including testing methods, supplier qualifications, and regulatory submission tips.
Regulatory Standards for Growth Media
Standards and Guidelines
Growth media, a critical component in cultivated meat production, must meet rigorous international regulatory standards. These standards vary across regions, each with specific requirements for composition, safety, and purity.
In the United Kingdom, growth media is regulated under the Novel Food Regulations (assimilated Regulation (EU) 2015/2283). Before being approved for the market, a thorough safety assessment is required [1]. The Food Standards Agency (FSA) classifies cell-cultivated products as Products of Animal Origin (POAO) under Regulation (EC) 853/2004. This classification mandates that producers implement food safety management systems based on the Hazard Analysis and Critical Control Points (HACCP) principles [3]. The FSA is also in the process of developing detailed technical guidance on growth media composition, with further updates expected [1]. These frameworks provide the foundation for more specific regulatory requirements.
In the United States, the approach differs. Growth media components must meet the Current Good Manufacturing Practice (CGMP) requirements outlined in Section 501(a)(4)(B) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) [4]. The FDA categorises media components as "supplies and reagents", which are governed by 21 CFR parts 210 and 211. These components must undergo quality verification to prevent contamination [4]. Interestingly, synthetic components of cultivated meat media - such as amino acids, vitamins, and salts - are often classified as Class I medical devices under 21 CFR 864.2220, exempting them from premarket notification requirements [6][7].
In the European Union, the regulatory framework aligns closely with the UK's, as it also follows Regulation (EU) 2015/2283. The European Food Safety Authority (EFSA) oversees the authorisation process [1]. According to ICH Q6B guidelines, growth media components, including antibiotics, inducers, and other constituents, are treated as process-related impurities. These impurities must be controlled and reduced to acceptable levels [5]. Where possible, excipients and reagents should comply with pharmacopoeial standards [5].
| Jurisdiction | Primary Regulation | Classification | Safety System | Media Oversight |
|---|---|---|---|---|
| United Kingdom (GB) | Assimilated Regulation (EU) 2015/2283 [1] | Product of Animal Origin (POAO) [3] | HACCP (Reg 852/2004) [3] | FSA/FSS Sandbox Guidance [1] |
| European Union / NI | Regulation (EU) 2015/2283 [1] | Product of Animal Origin (POAO) [3] | HACCP (Reg 852/2004) [3] | EFSA Authorisation Process [1] |
| United States | FD&C Act Section 501(a)(4)(B) [4] | New Animal Drug / Food [4] | CGMP (21 CFR 210/211) [4] | FDA CVM / USDA-FSIS [4] |
Regulatory Requirements for Cultivated Meat
Producers of cultivated meat must ensure that every batch of growth media adheres to strict safety and quality standards. Growth media validation is a key aspect of the broader regulatory framework for these products. Under HACCP principles (Regulation (EC) 852/2004), growth media is identified as a primary input and a potential source of contamination - chemical, microbial, or otherwise [3]. The FSA highlights this concern:
"The main hazards in cell-cultivated products production concern cell line identity (and consistency), hazards introduced during production process (microbiological contamination, growth media and residual components in the final product), and allergens." [3]
If there are changes to the growth media formulation, an immediate HACCP review is required [3]. In the UK, validation must take place before implementation to ensure the accuracy of flow diagrams and the effectiveness of control measures [3].
In the United States, the FDA mandates that all reagents and media components meet stringent quality standards to avoid introducing harmful agents [4]. Suppliers and contract laboratories must adhere to CGMP regulations, and any supplier failing to comply should be removed to prevent products from being classified as "adulterated" [4]. The FDA underscores the importance of this:
"All new animal drugs, including ACTPs, must be manufactured in accordance with CGMP to ensure that such drugs meet the requirements of the Federal Food, Drug, and Cosmetic Act (FD&C Act) as to safety." [4]
Currently, several companies participating in the UK's regulatory sandbox - such as BlueNalu, Gourmey, Hoxton Farms, Mosa Meat, Roslin Technologies, Vital Meat, and Vow - are collaborating with the FSA to refine these technical standards [1]. Under UK regulations, businesses can request up to five years of data protection for confidential information submitted during the authorisation process [1].
Steps for Validating Growth Media
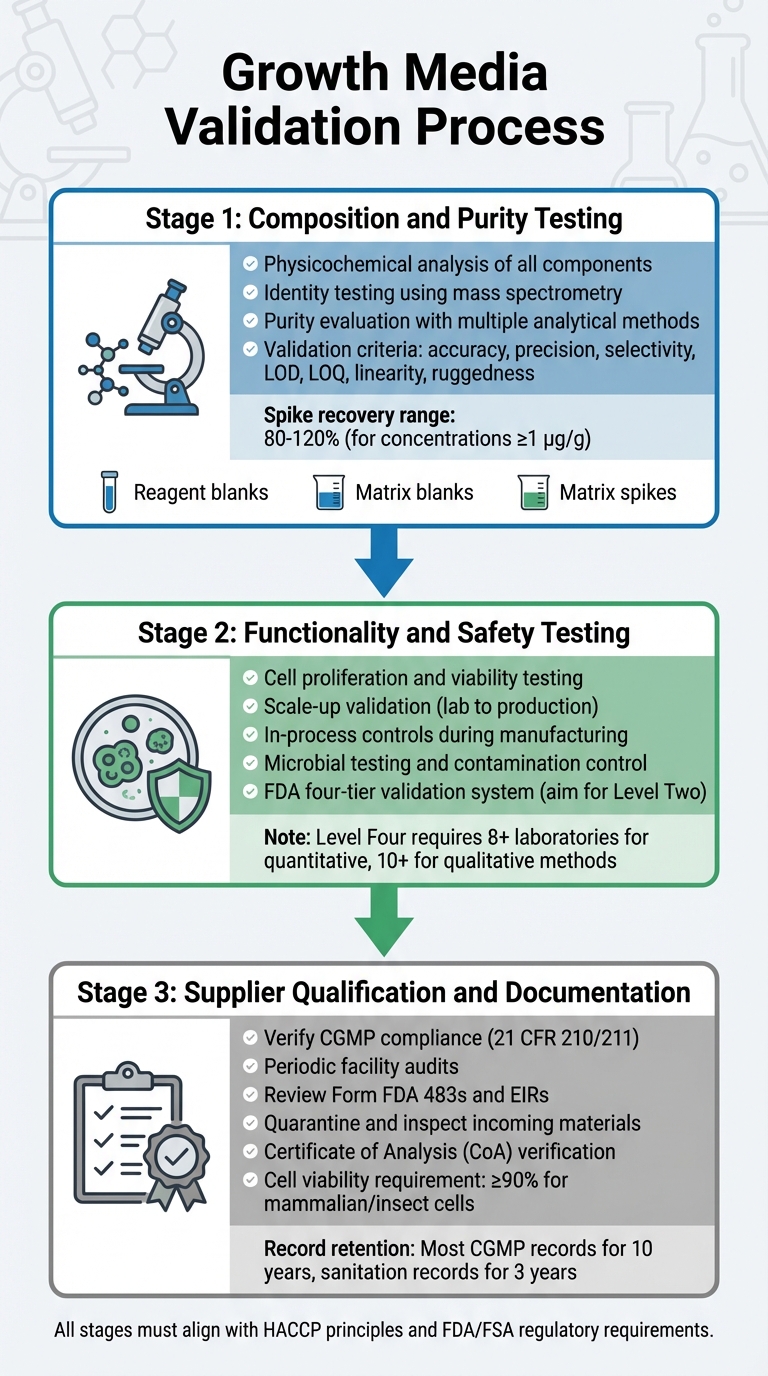
Growth Media Validation Process for Cultivated Meat Regulatory Approval
Validating growth media involves a detailed process that examines its composition, functionality, safety, and supplier compliance. Each step builds on the last, ensuring a robust validation process that aligns with regulatory requirements. This includes testing for composition, functionality, and bioprocess media supplier compliance.
Composition and Purity Testing
The first stage focuses on a thorough physicochemical analysis of each component. This involves identifying the precise composition, physical properties, and molecular structure of ingredients such as amino acids, vitamins, and inorganic salts [5]. To confirm molecular structures, identity testing employs highly specific methods, including physicochemical, biological, and immunochemical techniques. Tools like mass spectrometry are used to verify molecular identities through their fragmentation patterns [8].
Purity evaluation requires multiple analytical methods to separate desired components from impurities. These tests must address both process-related and product-related impurities [5]. Analytical techniques should meet strict validation criteria, including accuracy, precision, selectivity, limits of detection (LOD), limits of quantitation (LOQ), linearity, and ruggedness [8]. Validation protocols should also incorporate:
- Reagent blanks to ensure reagents are free from analytes.
- Matrix blanks to confirm the sample environment does not interfere.
- Matrix spikes to estimate recovery and accuracy.
For quantitative methods at concentrations of 1 µg/g (ppm), acceptable spike recoveries typically range between 80% and 120% [8].
To maintain consistency, manufacturers should establish in-house primary reference materials derived from production-representative lots. These serve as traceable standards for calibrating working reference materials [5]. Once purity testing is complete, the media must demonstrate its ability to support effective cell growth and meet safety standards.
Functionality and Safety Testing
After confirming the composition, the media must prove its effectiveness in supporting cultivated meat production. This includes demonstrating that cells can proliferate, maintain viability, and scale from laboratory conditions to production volumes. This transition often requires pilot-scale systems to generate the necessary regulatory data. The FDA requires in-process controls during manufacturing, starting from early stages like cell passaging and harvesting, to ensure product consistency and safety [4].
Safety validation involves rigorous microbial testing and contamination control, as outlined in the FDA's pre-market evaluations [9].
The FDA uses a four-tier system for chemical method validation, ranging from Level One (emergency or limited use) to Level Four (full collaborative studies meeting AOAC/ISO standards) [8]. For routine regulatory testing, aim for Level Two single-laboratory validation, which includes a comprehensive performance evaluation [8]. Full collaborative studies for quantitative methods require participation from at least eight laboratories, while qualitative methods need ten [8]. Once the media's performance is validated, it's essential to ensure that all raw materials come from compliant suppliers.
Supplier Qualification and Documentation
Manufacturers must work with verified, CGMP-compliant suppliers. Suppliers should meet the standards outlined in 21 CFR 210/211 [4]. Verification involves periodic audits of supplier facilities to assess adherence to quality programmes, procedures, and overall CGMP compliance [4].
Before entering into contracts, review a supplier's compliance history, including Form FDA 483s and Establishment Inspection Reports (EIRs) [4]. The FDA underscores this obligation:
"Before entering into any contract, agreement, or other arrangement with another establishment to perform any manufacturing step for you, you should verify that the establishment complies with applicable regulatory CGMP." [4]
All incoming materials must be quarantined and inspected before release, ensuring they meet master specifications [10]. Suppliers are required to provide a Certificate of Analysis (CoA) or traceable, CGMP/GLP-compliant test results [10]. For stable cell lines, documentation must include a traceable cloning history [10]. Mammalian or insect cells typically require at least 90% viability for acceptance in CGMP projects [10]. Records should be retained as per regulatory guidelines [4].
Contracts must clearly outline CGMP responsibilities and require suppliers to notify manufacturers of any proposed changes to test kits or methodologies [4]. If testing is outsourced, ensure that contract laboratories use validated analytical methods and are FDA-registered [4].
Preparing Regulatory Submission Documents
Once your growth media has been validated, the next step is to compile a dossier that demonstrates compliance with all safety and quality standards required by the FDA and USDA-FSIS. This dossier serves as a critical link between validation and regulatory compliance, giving authorities a clear view of your media's safety and production processes.
Required Elements of a Submission Dossier
Your dossier should include a detailed breakdown of the media composition, listing all amino acids, vitamins, inorganic salts, and growth factors. FDA guidelines emphasise that the review process evaluates not just the media itself but the entire production workflow. This includes the establishment of primary and immortalised cell lines and banks, implementation of manufacturing controls, and verification of all components and inputs [11].
Additionally, the dossier must feature a thorough safety and toxicological assessment, proving the food safety of the cultured material and all its inputs. Include manufacturing control records, process validation data, and quality programme documentation to demonstrate that your production is consistent and free from contaminants.
You should also provide supply and reagent verification records, showing validation for all materials used in the media, including those prepared in-house. For products regulated by the USDA-FSIS, include HACCP plans and sanitation protocols. The FDA advises retaining most CGMP records for at least 10 years, while facility cleaning and sanitation records should be kept for a minimum of 3 years [4]. This aligns with supplier qualification efforts, ensuring all inputs meet CGMP and regulatory requirements.
Documenting Facility Compliance
Before producing, processing, or storing cultivated meat for human consumption, facilities must register with the FDA [12]. Your documentation should include a comprehensive food safety plan that addresses hazard analysis (biological, chemical, and physical), preventive controls (such as sanitation, allergen management, and supply chain measures), and oversight procedures [12].
Media fill simulations are also a key requirement. These involve 14-day incubation and growth promotion testing to confirm aseptic practices. As the FDA explains:
"The media fill should evaluate the aseptic assembly and operation of the critical (sterile) equipment, qualify the operators and assess their technique, and demonstrate that the environmental controls are adequate" [2].
Ensure your records include supplier qualification data, such as tests conducted on the first three batches of medium from a vendor to confirm they match the Certificate of Analysis. Other essential records include environmental control logs, equipment calibration schedules, and temperature monitoring data. For USDA-regulated processes, prepare HACCP plans, written sanitation standard operating procedures (SSOPs), and recall procedures [12][13].
sbb-itb-ffee270
Using Cellbase for Regulatory-Compliant Growth Media Procurement
Verified Suppliers for Cultivated Meat
Once you've validated your growth media formulation, the next step is sourcing components that meet regulatory standards. This isn’t as simple as ordering from generic suppliers. For cell-cultivated products, strict hygiene regulations apply, and every growth media component must come with specific documentation for regulatory approval [3]. That’s where Cellbase steps in. It connects cultivated meat companies with suppliers who are not only verified but also well-versed in these compliance requirements, ensuring they provide the necessary paperwork.
Cellbase helps companies align with Food Standards Agency guidelines by ensuring suppliers follow robust quality control systems. This guarantees that all inputs meet regulatory standards, while also supporting the traceability and documentation required under general food law [3].
Procurement Features
Cellbase offers a searchable product catalogue, allowing procurement teams to easily find growth media components with essential specifications like serum-free or GMP-compliant labelling. This feature not only speeds up the procurement process but also ensures that all components meet the strict standards set by UK regulators.
The platform also provides transparent pricing and a direct messaging feature, enabling teams to quickly request quotes, certificates of analysis, and other regulatory documents. By consolidating these critical procurement functions into one system tailored for cultivated meat production, Cellbase simplifies the process and reduces the administrative workload of maintaining a compliant supply chain.
Conclusion
Validating growth media for regulatory approval isn't just a box to tick - it's a legal requirement for introducing cultivated meat products to the UK market. This involves thorough testing for composition and purity, implementing a strong HACCP plan, and keeping detailed documentation every step of the way.
"Food must not be placed on the market if it is unsafe. This means that it is neither injurious to health nor unfit for human consumption." - Food Standards Agency [3]
The UK Food Standards Agency's £1.6 million Regulatory Sandbox highlights its commitment to working with industry players to establish clear technical guidance on growth media composition [1]. Companies that prioritise proper validation now will be in a stronger position when these guidelines are fully defined.
Meeting compliance standards isn't just about ticking regulatory boxes - it’s about earning consumer trust and ensuring product safety. Rigorous quality testing is at the heart of both regulatory approval and gaining market acceptance. To streamline the authorisation process, focus on building strong validation protocols, maintaining accurate records, and partnering with reliable suppliers. These steps will not only simplify approval but also pave the way for greater consumer confidence.
FAQs
What are the main steps to validate growth media for regulatory approval?
Validating growth media for regulatory approval is all about proving that the formulation is safe, reliable, and suitable for producing cultivated meat. Here's what the process usually looks like:
- Risk assessment: Start by defining the cell line you'll use, the product's goals, and its critical quality attributes (like pH or nutrient composition). Identify any potential hazards, such as microbial contamination, and lay out measures to control these risks.
- Testing and specifications: Set clear acceptance criteria for factors like sterility, purity, and potency. Use established testing methods to ensure these standards are consistently met.
- Validation studies: Conduct thorough process validation, including qualifying equipment and testing multiple batches, to confirm that results are reproducible and consistent.
- Stability testing: Check how the media holds up over time by assessing its quality throughout its intended shelf life under proper storage conditions (typically 2–8 °C).
- Documentation: Pull everything together into a comprehensive validation dossier. This should include all test results and analyses to satisfy regulatory requirements.
By carefully addressing each of these steps, you'll gather the evidence needed to show the media meets the safety and quality standards required for cultivated meat production.
What are the key differences between UK and US regulations for growth media used in cultivated meat?
In the United Kingdom, the regulation of growth media for cultivated meat falls under the Novel Foods Regulation (EU Regulation 2015/2283), which has been retained in GB law. Any growth media used in products not commonly consumed before 15 May 1997 must go through a formal novel-food assessment by the Food Standards Agency (FSA). This process requires submitting detailed documentation, including information on the media’s composition, origin, and purity. Additionally, a HACCP-based risk assessment is necessary to demonstrate how contaminants are controlled during the cell-culture process.
Since December 2025, the FSA has implemented a Cell-Cultivated Products sandbox. This initiative offers guidance and supports faster data collection for novel-food applications. To gain final authorisation, companies must submit a comprehensive dossier that addresses media safety, consistency, and manufacturing validation. Only after this approval can the product be sold in Great Britain.
In contrast, the United States does not have a specific novel-food framework tailored to growth media, making direct regulatory comparisons challenging. For UK-based companies, sourcing media components that already comply with these strict standards can simplify the approval process. Cellbase provides a reliable marketplace where researchers and producers can find growth media verified to meet UK regulatory requirements, helping to smooth out the validation and approval workflows.
How does the UK's regulatory sandbox support growth media validation?
The UK’s regulatory sandbox for cultivated products provides a well-organised setting where companies can test and refine their growth media formulations. Overseen by the Food Standards Agency (FSA) and Food Standards Scotland (FSS), this programme runs in six-month phases. During this time, businesses can carry out safety tests, perform risk assessments, and review documentation while receiving valuable feedback from regulators.
This hands-on approach allows for practical trials and step-by-step improvements, speeding up the collection of safety data and helping companies align with regulatory requirements. For those working on cultivated meat, sourcing pre-approved growth media through Cellbase can make the process smoother. It ensures materials meet the FSA’s strict standards and that all required records are prepared for regulatory submissions.











