Cleaning validation is critical in cultivated meat production to prevent contamination and ensure product safety. Here's what you need to know:
- Regulatory Standards: Cleaning processes must remove 99% of microorganisms, followed by disinfection or sterilisation achieving 99.999% reduction.
- Residue Challenges: Bioreactors accumulate proteins, fats, and cellular debris, requiring precise cleaning methods. Single-use systems add risks like hydrocarbons and siloxanes.
-
Key Tools for Residue Detection:
- HPLC: Detects specific residues but has sensitivity limitations for trace contaminants.
- LC-MS/MS: Highly sensitive, detecting ng/mL levels, ideal for trace analysis.
- TOC Analysis: Measures all organic residues quickly (ppb sensitivity) but lacks specificity.
- Microbial Detection: Traditional sterility tests are slow (5–7 days). Rapid methods like ATP bioluminescence and real-time PCR provide faster results, improving batch release timelines.
- Digital Monitoring: Real-time tools like UV spectroscopy and AI-driven analytics optimise cleaning cycles, reduce downtime, and improve efficiency.
Novel Analytical Methods to Verify Cleaning Process
Residue Detection Tools
In cultivated meat production, cleaning bioreactors is a meticulous process. Residues like proteins, fats, cellular debris, and growth media components must be completely removed to avoid cross-contamination. Tools like HPLC, LC-MS/MS, and TOC analysis each play a role in ensuring thorough residue detection, offering both quantitative and qualitative insights.
High-Performance Liquid Chromatography (HPLC)
HPLC is a widely used method for measuring residues in bioreactors. When paired with ultraviolet (UV) detection, it helps separate and identify components in liquid samples. This makes it particularly useful for quantifying stable residues, such as specific growth media components or cleaning agents. However, it has limitations. For instance, HPLC-UV might not be sensitive enough to detect trace residues, especially in applications involving high-potency peptides that are prone to adsorptive loss or have poor UV sensitivity [3].
Typically, HPLC-UV achieves detection limits in the µg/mL range, which might not suffice for monitoring minor contamination. Still, its reliability in detecting and validating the removal of certain residues makes it a go-to method for ensuring product safety in cultivated meat processes [3].
Mass Spectrometry Techniques
LC-MS/MS takes residue detection to the next level with its heightened sensitivity and specificity. This method can analyse a wide range of peptides, detecting quantities as low as 1–1,000 ng/mL in a single run. By using multiple reaction monitoring fragments, it confirms the identity of residues with precision. As noted by Waters Corporation:
While High Performance Liquid Chromatography (HPLC) coupled to Ultraviolet (UV) detection is the most common analytical tool for ARL determination, there is a growing need for analytical methodologies which can achieve more sensitive and selective detection [3].
LC-MS/MS is particularly effective for identifying trace residues, degraded proteins, and extractables from single-use bioreactor components. Analysts often rely on high-performance surface vials to minimise non-specific binding and improve recovery rates. Its ability to detect residues at extremely low levels (ng/mL) makes it indispensable for confirming the removal of high-potency ingredients from bioreactor surfaces [3].
Total Organic Carbon (TOC) Analysis
TOC analysis measures the total organic carbon in residues by oxidising them into CO₂ and monitoring the change in conductivity. This method is non-specific, meaning it detects all organic residues - whether they are proteins, cells, cleaning agents, or media components. Its sensitivity is impressive, with detection limits as low as 6.30 ppb and quantitation limits around 21 ppb [4][5].
A study from the Center for Genetic Engineering and Biotechnology in Havana, Cuba, demonstrated the effectiveness of TOC analysis. Researchers achieved a three-order magnitude reduction in residue levels, with final TOC values as low as 22 ppb. They also established a link between TOC readings and microbial load: for example, 27 ppb of TOC correlated to approximately 10⁶ E. coli cells, while 16 ppb equated to roughly 10³ yeast cells [4].
TOC analysers are particularly well-suited for Clean-In-Place systems, where they can be used as at-line or on-line tools to speed up equipment turnaround times [5]. The European Commission's Annex 15 supports the use of non-specific methods like TOC when specific residue testing isn’t feasible, stating:
Biologics are known to degrade and denature when exposed to pH extremes and/or heat... [supporting] non-specific methods, such as total organic carbon (TOC) and conductivity, when it is not feasible to test for specific product residues [5].
While TOC analysis cannot differentiate between residue types - such as growth media, cellular debris, or cleaning agents - this broad detection is beneficial for validating the removal of degraded proteins. For large-scale cell cultures, the correlation between TOC and cell count offers a practical way to confirm biomass removal from bioreactor walls [4].
Together, these tools provide a robust framework for residue detection, ensuring bioreactors meet the stringent cleanliness standards required for cultivated meat production. This foundation is crucial for subsequent sterility and microbial testing.
Sterility Testing and Microbial Detection
After residue detection, ensuring sterility is absolutely critical. Traditional sterility tests often take 5–7 days for microbial colonies to grow to detectable levels (about 10⁷ cells) [8]. This lengthy process can delay equipment turnover and batch release in cultivated meat production. However, rapid microbial methods (RMM) can significantly cut this waiting time, detecting contamination in hours rather than days. Let’s take a closer look at these methods.
One major hurdle in bioreactor cleaning validation is the difficulty of culturing certain organisms with standard techniques. For instance, in September 2023, AstraZeneca used amplified ATP bioluminescence to quickly identify slow-growing organisms like Dermacoccus nishinomiyaensis, which standard tryptic soya agar couldn’t detect. This highlights how rapid methods outperform traditional culture techniques. As Miriam Guest, Principal Scientist at AstraZeneca, explained:
"...enabling a fast response to ensure mitigations could be executed in a timely manner."
– Miriam Guest, Principal Scientist, AstraZeneca [6]
Automated systems further improve accuracy by eliminating human error during manual readings. They also integrate directly with Laboratory Information Management Systems (LIMS), reducing transcription errors and speeding up documentation - a huge advantage for cultivated meat facilities managing multiple batches [8].
Rapid Microbial Detection Methods
To overcome the limitations of traditional culture methods, several rapid detection technologies have emerged. Here’s how they work:
- ATP Bioluminescence: This method detects adenosine triphosphate (ATP) from living cells, providing results within minutes to hours. While non-specific, it’s excellent for quick hygiene checks and can identify organisms that agar plates might miss [6][7].
- Nucleic Acid-Based Methods: Techniques like real-time PCR and LAMP (loop-mediated isothermal amplification) offer high sensitivity and specificity. Real-time PCR can detect as few as 10⁴ cfu/mL in 1–3.5 hours after enrichment [7]. LAMP, operating at a steady temperature (59–65°C), delivers results in 60–75 minutes post-enrichment, detecting between 10² and 10⁴ cfu/mL. Reverse-transcription LAMP (rtLAMP) for RNA detection achieves even greater sensitivity, identifying as few as 4 cfu per swab without enrichment [7].
- Optical Assays: These rely on broth media containing dyes that change colour or fluoresce based on microbial metabolic activity. Platforms like BioLumix and Soleris can detect as few as 8 yeast cells or 50–100 bacteria - far lower thresholds than visual colony inspection [8]. Detection times range from 8–18 hours for a single bacterium and 35–48 hours for mould cells [7].
- Impedance Microbiology: This method monitors electrical changes in culture media caused by bacterial metabolism. It distinguishes between live and dead cells, delivering results in 14–24 hours [7].
When choosing a rapid method, one key factor to consider is whether the process is destructive. Fluorescence-based methods are often non-destructive, allowing for colony traceability, while ATP bioluminescence and cell lysis methods typically destroy the sample [8]. For bioreactor cleaning validation, where residual detergents or sanitisers might interfere, pre-moistening swabs with neutralising agents can help avoid false negatives [7].
sbb-itb-ffee270
Digital and Process Analytical Tools
The introduction of Process Analytical Technology (PAT) and digital monitoring platforms is transforming cleaning validation in cultivated meat production. Traditionally, offline testing meant equipment had to sit idle for hours - or even days - while waiting for lab results [9]. Now, in-line and online tools provide real-time data throughout the cleaning cycle, eliminating these delays.
Take in-line UV spectroscopy as an example. This technology uses sensors to monitor cleaning agents and protein residues in real time. As John Schallom from STERIS explains:
The in-line monitoring capability of UV enables real-time continuous monitoring of the entire cleaning cycle and applicability to quality by design, process analytical technology process digitalization, and sustainability goals of a Pharma 4.0 manufacturing facility. [5]
By using tools like UV spectroscopy and UPLC, residue levels are measured with precision during the cleaning process. This enables a "clean until clean" approach, where washing stops as soon as residue levels meet the target thresholds, rather than relying on fixed cleaning times designed for worst-case scenarios. The result? Equipment downtime is drastically reduced [9]. These continuous monitoring systems also pave the way for predictive cleaning protocols, improving efficiency and reducing waste.
AI-Driven Predictive Analytics
AI is playing a key role in optimising cleaning protocols. Through digital twins, AI simulates TACT (Temperature, Action, Chemistry, Time) variables, streamlining the process by reducing the need for repeated experiments. Machine learning analyses the interplay of these variables to identify the most efficient and reproducible cleaning conditions [11]. This approach not only saves time and resources but also supports efforts to make cultivated meat more cost-competitive with traditional meat [10].
Real-Time Monitoring Platforms
Real-time monitoring platforms combine multiple sensors to continuously verify cleanliness throughout the cleaning cycle. For instance, in May 2014, Waters Corporation showcased the PATROL UPLC Process Analysis System. This system monitored wash solvents from a 1-litre reaction vessel using a 60-second isocratic method, achieving a cycle time of 160 seconds between injections with a detection limit of 24 ng/mL. This near-instant analysis eliminates the need for manual swabbing and reinforces the "clean until clean" methodology [9].
For cultivated meat facilities, these platforms deliver even greater benefits. Total Organic Carbon (TOC) analysis can detect as few as 1,000,000 E. coli cells at levels as low as 27 ppb [4], offering a sensitive method for assessing microbial cleanliness. Additionally, Surface Plasmon Resonance (SPR) technology provides a detection sensitivity between 1–10 ng/mL [2], making it invaluable for validating the cleaning of highly potent biologics. By integrating these real-time tools, cultivated meat producers can ensure efficient cleaning validation that aligns with stringent regulatory requirements.
For companies looking to adopt these cutting-edge solutions, Cellbase offers a wide range of trusted sensor technologies and critical instruments tailored to the needs of cultivated meat production.
Tool Comparison
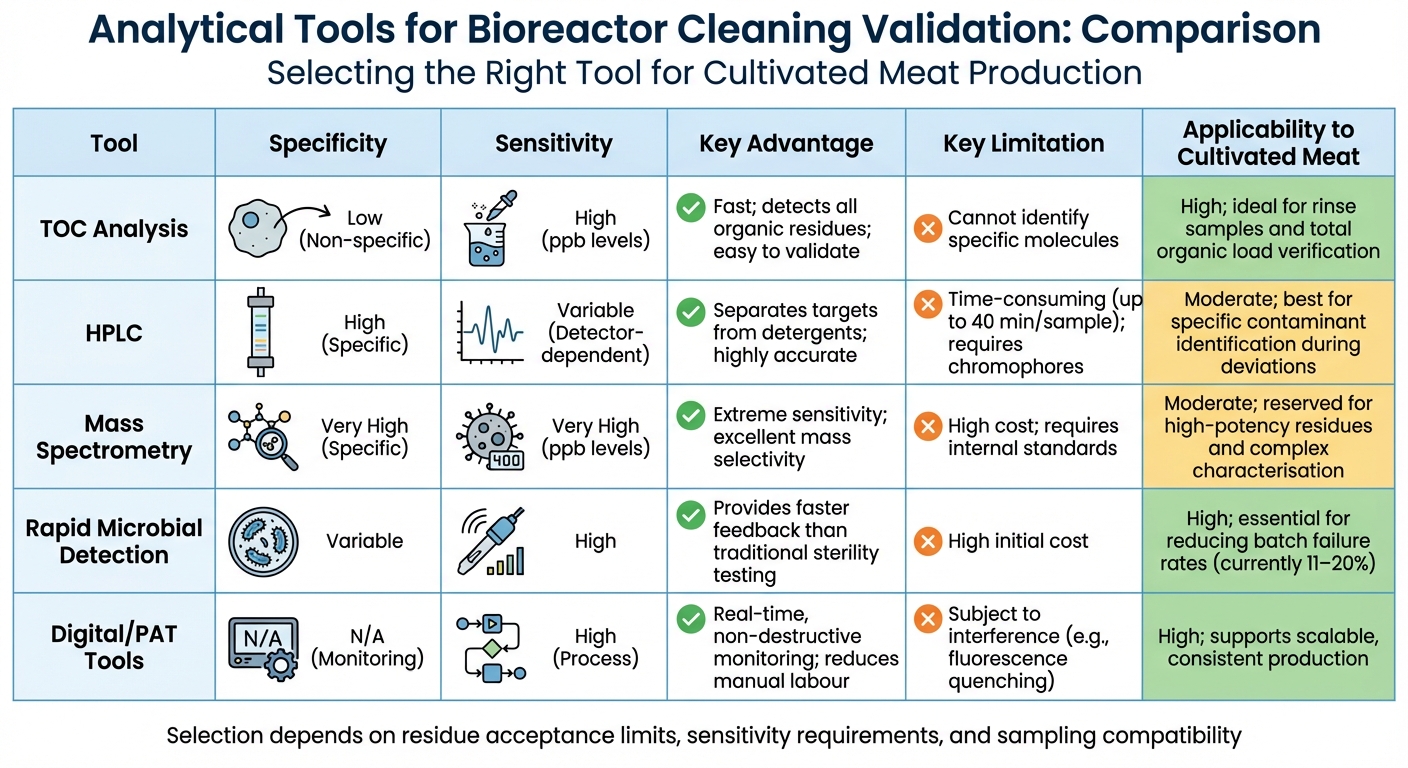
Comparison of Analytical Tools for Bioreactor Cleaning Validation in Cultivated Meat Production
Selecting the right analytical tool for bioreactor cleaning validation involves weighing factors like sensitivity, specificity, cost, and how well it fits into the cultivated meat production process. Here's a breakdown of how different tools contribute to this rigorous validation effort.
TOC analysis is a standout for its speed and ability to detect all organic residues, though it doesn't differentiate between specific molecules. It provides quick and sensitive verification of total organic load, making it particularly valuable for rinse water analysis, where confirming complete organic removal is key. However, because it measures total carbon, it cannot identify the specific types of organic matter present.
HPLC excels in specificity, as it separates target residues from detergents and other components in a single run. Its sensitivity depends on the chemical properties of the molecule and the type of detector used (e.g., UV or fluorescence). The downside? HPLC is time-intensive, taking up to 40 minutes per sample, not to mention the extensive preparation required before analysis[12]. While not ideal for routine monitoring, it’s highly effective for identifying contaminants during deviations.
Mass spectrometry offers unmatched specificity and sensitivity, capable of detecting molecules at extremely low levels (ppb). This makes it perfect for validating the removal of potent growth factors or proteins. However, it often requires an internal standard to ensure accuracy near residue acceptance limits. The high cost and complexity of mass spectrometry make it less practical for routine use, but it’s indispensable for investigating deviations or validating worst-case scenarios.
Comparison Table
The following table summarises the strengths and limitations of various tools used for residue detection and microbial monitoring. Each tool plays a distinct role in maintaining validated cleaning protocols.
| Tool | Specificity | Sensitivity | Key Advantage | Key Limitation | Applicability to Cultivated Meat |
|---|---|---|---|---|---|
| TOC Analysis | Low (Non-specific) | High (ppb levels) | Fast; detects all organic residues; easy to validate | Cannot identify specific molecules | High; ideal for rinse samples and total organic load verification[4][15] |
| HPLC | High (Specific) | Variable (Detector-dependent) | Separates targets from detergents; highly accurate | Time-consuming (up to 40 min/sample); requires chromophores | Moderate; best for specific contaminant identification during deviations[12][15] |
| Mass Spectrometry | Very High (Specific) | Very High (ppb levels) | Extreme sensitivity; excellent mass selectivity | High cost; requires internal standards | Moderate; reserved for high-potency residues and complex characterisation |
| Rapid Microbial Detection | Variable | High | Provides faster feedback than traditional sterility testing | High initial cost | High; essential for reducing batch failure rates (currently 11–20%)[14] |
| Digital/PAT Tools | N/A (Monitoring) | High (Process) | Real-time, non-destructive monitoring; reduces manual labour | Subject to interference (e.g., fluorescence quenching) | High; supports scalable, consistent production[13][15] |
This comparison highlights the need for a balanced approach that combines speed, specificity, and real-time monitoring. For cultivated meat facilities, which operate on tighter budgets than pharmaceutical manufacturers, TOC analysis often emerges as the most practical choice for routine validation. It demands far less method development compared to HPLC or mass spectrometry[12].
Conclusion
Combining residue detection with real-time monitoring is crucial for effective bioreactor cleaning validation in cultivated meat production. By leveraging analytical methods like TOC analysis, HPLC, and mass spectrometry, producers can address both routine checks and detailed deviation investigations. Each tool brings unique strengths to the table, ensuring a robust and comprehensive validation process.
The industry's move towards automated systems and real-time monitoring is a game-changer. These advancements minimise downtime and reduce batch failures, streamlining operations. As Ferdinand Groten aptly put it:
Automation increases the efficiency, stability, and reproducibility of the process and allows for consistent data documentation, therefore leading to a consistently high product quality and enabling up-scaling of the process yield [1].
Selecting the right tools involves considering residue acceptance limits, sensitivity, and sampling compatibility [12]. For high-potency proteins with strict Permitted Daily Exposure limits, Surface Plasmon Resonance technology offers exceptional sensitivity, detecting as low as 1–5 ng/mL - far surpassing the 90–95% degradation levels demonstrated by SDS-PAGE [2].
Sourcing reliable, biopharmaceutical-grade analytical equipment is no small task. Platforms like Cellbase simplify this by connecting producers with validated suppliers specifically suited for cultivated meat production. This not only keeps validation timelines on track but also ensures compliance with rigorous documentation and quality standards required by regulators.
The key to success lies in a validation strategy that balances speed, precision, and scalability. Rapid routine monitoring must work hand-in-hand with the capacity for in-depth investigations when needed. Coupled with efficient equipment sourcing, this approach ensures consistent, compliant processes that meet the demands of scalable cultivated meat production.
FAQs
How do I choose between TOC, HPLC and LC-MS/MS for cleaning validation?
When deciding between TOC, HPLC, and LC-MS/MS, it all comes down to what you need to detect and how precise the method has to be.
- TOC (Total Organic Carbon): This method measures overall organic residues, such as detergents, but it doesn't pinpoint specific compounds. It's a broad approach, useful for general residue monitoring.
- HPLC (High-Performance Liquid Chromatography): This is a more targeted option, perfect for identifying and quantifying known impurities in your samples.
- LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry): If you're after extreme sensitivity or need to analyse complex samples, this is the go-to method. It excels at detecting trace residues down to minute levels.
The right choice depends on your process requirements and the nature of the residues you're dealing with.
What are the residue acceptance limits for a bioreactor?
Residue acceptance limits for a bioreactor are set based on health-based exposure levels, like acceptable carryover or permitted daily exposure (PDE) values. These limits are crucial to ensure patient safety while meeting regulatory standards, in line with established guidelines.
What is the best rapid microbial method when sanitisers may interfere?
The 7000RMS Microbial Detection Analyzer is a great choice for situations where sanitisers might affect results. It provides continuous bioburden monitoring, capturing data every two seconds. This helps reduce the impact of sanitiser interference, delivering consistent and dependable outcomes.











