ISO 14644 sets the standards for cleanroom air quality, crucial for industries using cultivated meat production systems. The guidelines cover particle limits, monitoring strategies, and contamination control methods. Here's what you need to know:
- ISO 14644-1: Defines cleanliness classes (ISO 1 to ISO 9) based on particle counts. For instance, ISO Class 5 allows up to 3,520 particles (≥0.5 µm/m³).
- ISO 14644-2: Focuses on risk-based monitoring, ensuring compliance under "at-rest" and "in-operation" conditions.
- Key Metrics: Monitor particle counts, pressure differentials (10–15 Pascals), temperature (18–22°C), and humidity (30–60%).
- Methods: Use Light Scattering Airborne Particle Counters (LSAPC), microbial air sampling, and surface testing to detect contamination.
- Automation: Continuous monitoring systems provide real-time data, reducing risks and improving compliance with regulatory standards like FDA 21 CFR Part 11.
Proper monitoring prevents contamination, protects products, and ensures compliance with cleanroom standards.
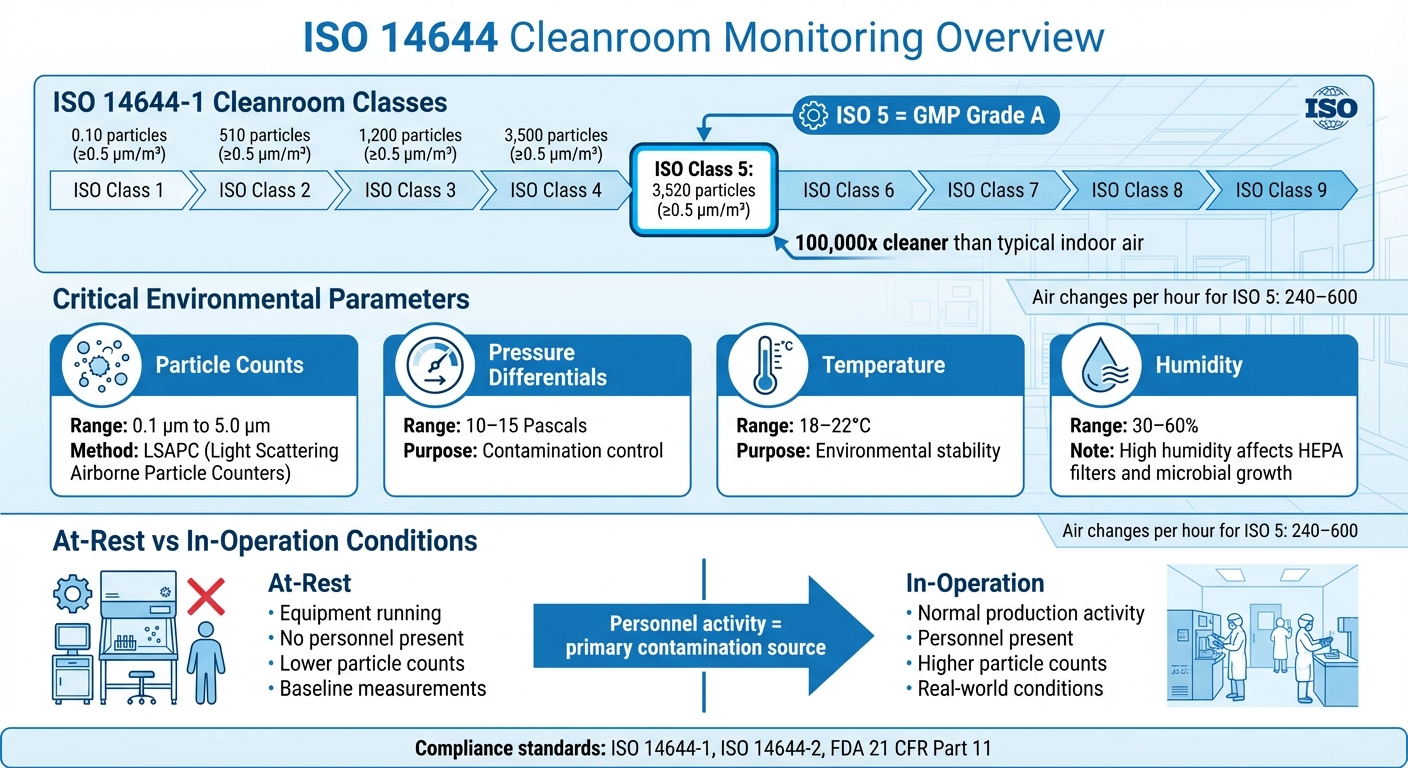
ISO 14644 Cleanroom Classification Standards and Key Monitoring Parameters
Creating a Risk-Based Monitoring Plan
Conducting Risk Assessments
When it comes to cleanroom monitoring, taking a risk-based approach ensures that the monitoring process aligns closely with the actual conditions of operation. This isn't about following generic templates - it's about tailoring the plan to the specific risks of your environment.
According to ISO 14644-2:2015, monitoring plans must be rooted in formal risk assessments [3][4]. Tools like HACCP and FMEA are particularly useful for systematically identifying contamination risks and pinpointing critical control points where cultivated meat products come into direct contact with the cleanroom environment. It’s also crucial to differentiate between "at-rest" conditions (when equipment is running without personnel) and "in-operation" states (during normal production), as personnel activity can significantly increase particle levels [1].
Don’t overlook adjacent spaces like airlocks, gowning rooms, and corridors. These areas play a vital role in maintaining proper pressure cascades, which are essential for contamination control. Regulatory guidance also emphasises the need for monitoring plans that include these adjoining spaces and incorporate batch-specific environmental checks to mitigate contamination risks.
Selecting Monitoring Locations and Parameters
Choosing the right locations for sensors is key to effective monitoring. Start by mapping all classified areas and supporting zones. Sensors should be strategically placed to gather representative data without interfering with operations. Prioritise areas where cultivated meat is exposed, personnel entry points, and spaces adjacent to lower-classified zones.
A robust monitoring plan should go beyond just counting particles. It should also track key parameters like airborne particle sizes (ranging from 0.1 µm to 5 µm), air pressure differentials, temperature (typically 18–22°C), and relative humidity (30–60%). High humidity levels can encourage microbial growth and even reduce the efficiency of HEPA filters [1][3].
sbb-itb-ffee270
Intro to ISO 14644-2 {Part 1} - Cleanroom Monitoring Plan (2019)
Airborne Particle Monitoring Procedures
Accurate airborne particle monitoring starts with a solid risk assessment and is supported by precise procedures that ensure reliable data collection and timely responses.
Operating Airborne Particle Counters
To capture accurate particle data, use Light Scattering Airborne Particle Counters (LSAPC) that meet ISO 21501-4 standards. These devices should be calibrated with NIST-traceable particles for reliable measurements. Place sensors at critical control points, ensuring they don’t obstruct cleanroom traffic or disrupt airflow. For accurate readings, position the probe within 30 cm of the work area, aligning it with the airflow to maintain isokinetic sampling conditions.
It’s important to note that particle counts differ significantly between "at-rest" conditions (equipment running, no personnel present) and "in-operation" states, where activity increases particle levels. Moving from periodic classification to continuous monitoring is key to detecting short-lived spikes that manual testing might overlook [1].
This methodical approach naturally supports setting clear thresholds for action.
Establishing Alert and Action Levels
Once sensors are in place, defining alert and action levels is essential to minimise contamination risks.
Thresholds should be based on a risk-based strategy, rather than simply adopting ISO classification limits. Alert levels act as early warnings, flagging deviations from normal conditions. Action levels, on the other hand, are set at the maximum particle concentration allowed for your ISO Class and require immediate investigation and corrective action. For instance, ISO Class 5 environments limit particle counts to no more than 3,520 particles (≥0.5 µm) per cubic metre, making them about 100,000 times cleaner than typical indoor air [1]. By setting alert levels below these limits, you create a buffer to investigate potential issues like gradual HEPA filter degradation or seal failures.
Every decision regarding thresholds should be documented in your monitoring plan. This includes the reasoning behind each level and the corresponding response procedures. Additionally, combining particle data with other environmental metrics - such as pressure differentials, temperature, and humidity - helps pinpoint factors contributing to contamination incidents.
Microbial Monitoring Methods
In addition to airborne particle monitoring, microbial testing plays a key role in detecting live contaminants that can affect cultivated meat production.
Airborne particle counters focus on identifying non-living particles, but microbial monitoring is necessary to uncover living organisms that could compromise cleanroom environments. While ISO 14644 provides guidelines for particle classification, cultivated meat facilities must also manage microbiological limits, particularly in critical zones where products are exposed.
Active and Passive Air Sampling
Active air sampling involves using microbial air samplers to pull a specific volume of air onto culture media, providing results in CFU/m³. This method allows for precise control over sampling location and volume, making it ideal for validating critical areas during performance qualification. On the other hand, passive sampling uses settle plates left exposed for 1–4 hours to monitor environmental trends with minimal equipment.
In ISO 5 critical areas, which align with GMP Grade A standards, microbial limits are exceptionally stringent. The US FDA’s 2004 Aseptic Processing Guidance highlights this, stating:
Samples from Class 100 (ISO 5) environments should normally yield no microbiological contaminants [6].
Any microbial presence in ISO 5 areas demands immediate investigation and a thorough root cause analysis.
Together, active and passive sampling methods form the foundation for effective surface monitoring.
Surface Sampling Techniques
Surface sampling is a crucial addition to air monitoring, focusing on detecting contamination on work surfaces, equipment, and other critical areas. Contact plates (RODAC), usually 55 mm in diameter, are pressed against smooth surfaces for about 10 seconds to transfer microorganisms directly onto the culture medium, providing measurable results. For irregular or hard-to-reach surfaces, swab sampling is more effective. Pre-moistened sterile swabs are used in a systematic "S" motion across defined areas (25–100 cm²) to ensure thorough and representative sampling [5].
Both methods require culture media with neutralising agents, such as Letheen broth, to counteract any residual disinfectants that could inhibit microbial growth and cause false negatives. Incubation conditions are tailored to the organism type: bacteria are incubated at 30–35°C, while fungi require 20–25°C for up to five days [5]. Post-cleaning verification, conducted after cleaning but before production begins, ensures the environment meets the required standards. As Vaibhavi M., an expert in the field, explains:
Surface monitoring forms the cornerstone of contamination control programmes in pharmaceutical cleanrooms [5].
Automated Environmental Monitoring Systems
Automated systems provide a steady stream of real-time data on factors like particle counts, pressure, temperature, and humidity. This continuous monitoring captures fleeting contamination events that periodic testing might miss, offering a valuable complement to manual methods.
The 2015 revision of ISO 14644-2 highlights the benefits of automated monitoring, particularly in enabling data-driven requalification. By reliably capturing data that meets regulatory standards, these systems can help extend the intervals between formal classification tests, ultimately reducing costs [7].
A cautionary example comes from June 2024, when the FDA issued a warning to Optikem International Inc. The company had relied solely on periodic monitoring, which failed to detect contamination events between February 2021 and March 2023. This oversight led to the facility being deemed unsuitable for sterile drug production [1].
Setting Up Continuous Monitoring Systems
When implementing an automated monitoring system, it’s essential to ensure that all airborne particle counters comply with ISO 21501-4 standards and support FDA 21 CFR Part 11 for electronic records, including features like audit trails and electronic signatures [7]. The best systems offer real-time dashboards that monitor key parameters such as particle counts, pressure differentials (typically 10–15 Pascals), temperature (18–22°C), and humidity (30–60%) simultaneously [1].
Proper placement of monitoring probes is critical. Probes should be positioned within 305 mm (1 foot) of exposed products or critical work areas [7]. Larger cleanrooms require at least one sensor for every 100 m² of the background environment, with additional sensors in transition zones like airlocks. For areas with unidirectional airflow, isokinetic sample probes are recommended to ensure accurate sampling [7].
Configuring alerts based on historical data trends - rather than just maximum ISO limits - can improve system responsiveness. As EU GMP Annex 1 advises:
The Grade A zone should be monitored at such a frequency and with suitable sample size that all interventions, transient events and any system deterioration would be captured and alarms triggered if alert limits are exceeded [7].
Some systems even include interactive SOP maps to assist with probe placement. Integration with Building Management Systems (BMS) or SCADA platforms can centralise oversight and potentially reduce energy consumption by up to 10% [1].
Once installed, these systems become a seamless part of daily operations, enabling immediate action in response to environmental fluctuations.
Analysing Real-Time Monitoring Data
Real-time data analysis works hand in hand with both particulate and microbial monitoring protocols. By enabling immediate responses to contamination events, it can prevent minor issues from escalating. Analysing trends over time can also reveal gradual declines in HEPA filter performance or seal integrity, helping to address potential issues before they lead to classification failures [1]. Advanced software tools can even correlate particle spikes with specific activities, such as door openings or HVAC cycles, to identify root causes [1].
In Grade A/B zones (ISO 5), consecutive counts of particles ≥5.0 µm should prompt an investigation. EU GMP guidance states:
Consecutive or regular counting of low levels [of 5.0 µm particles] is an indicator of a possible contamination event and should be investigated [7].
Alert levels should be tiered, with protocols ranging from minor investigations to critical responses requiring production to halt [1]. Remote management features allow supervisors to review and approve data via web browsers, simplifying compliance documentation [7]. For those seeking a streamlined approach, Monitoring as a Service (MaaS) solutions are available, starting at £600 per month [1].
For cultivated meat facilities looking for tailored solutions, Cellbase offers a B2B marketplace connecting professionals with trusted suppliers of automated environmental monitoring systems.
Connecting Monitoring with Maintenance and Compliance
Environmental monitoring data shouldn't exist in a vacuum. The most effective cleanroom programmes link particle counts, pressure readings, and microbial recoveries with HVAC performance metrics and cleaning schedules. By doing this, raw data transforms into actionable insights, enabling better maintenance decisions and reinforcing compliance during audits.
Correlating Monitoring with HVAC and Cleaning
Integrating monitoring data with maintenance records strengthens compliance efforts and simplifies ongoing quality control.
Trend analysis plays a crucial role in predictive maintenance. Instead of reacting to sudden classification failures, continuous monitoring can spot gradual issues like declining HEPA filter performance or weakening seal integrity before they escalate into larger problems [1]. For instance, rising particle counts or pressure levels dipping below 10–15 Pascals may indicate HVAC system inefficiencies [1].
Aligning environmental data with operational events can help identify anomalies. In cultivated meat facilities, this alignment is essential for maintaining aseptic conditions. For example, tracking particle spikes alongside door openings, personnel movements, or equipment cycles can help maintenance teams pinpoint specific mechanical or procedural issues rather than resorting to broad system overhauls [1]. Additionally, increased humidity levels can compromise HEPA filter performance and encourage microbial growth, signalling a need for HVAC adjustments [1].
Microbial recoveries act as a direct measure of cleaning effectiveness. If air or surface sampling reveals elevated microbial counts, it might be necessary to increase cleaning frequency or revise sanitation protocols [8].
ISO Class 5 cleanrooms, which require 240–600 air changes per hour to maintain particle limits, benefit from monitoring systems integrated with Building Management Systems (BMS) or SCADA platforms. These integrations centralise oversight and help ensure critical parameters remain stable [1].
Recording and Reviewing Monitoring Data
Thorough documentation is essential for ISO compliance audits. This includes maintaining a monitoring plan, calibration records, and timestamped audit trails, as required by both ISO and FDA standards [1][3][7].
Systems that comply with FDA 21 CFR Part 11 ensure records meet ALCOA principles - Attributable, Legible, Contemporaneous, Original, and Accurate [7]. Automated platforms can create secure, encrypted databases where historical records cannot be deleted, preserving the integrity regulators demand. Features like remote approval allow supervisors to review and sign off on daily monitoring data through web browsers, streamlining compliance processes [7].
When reviewing data, it's important to focus on trends rather than isolated incidents. Gradual patterns of deterioration often reveal problems before they reach critical levels [1][2]. As Particle Measuring Systems points out:
Without measurement there is no control [2].
Organising data by critical control points - such as filling zones or specific equipment - rather than general room data makes investigations more targeted and efficient [7].
Consistent monitoring data demonstrating stable conditions can also support extending classification testing intervals, cutting operational costs without compromising compliance [1][2]. With over 30% of FDA citations linked to Quality Systems deficiencies [1], robust monitoring records provide a critical safeguard during inspections.
For cultivated meat facilities, Cellbase offers connections to verified suppliers who specialise in equipment and documentation systems tailored to the unique demands of cultivated meat production.
Conclusion
Implementing ISO 14644 monitoring in cultivated meat production requires a well-structured risk assessment. This process should identify critical control points, determine optimal sensor placement, and establish practical alert and action levels to ensure effective contamination control [9].
The move from periodic testing to continuous automated monitoring marks a major shift in cleanroom management. While ISO 14644-1 provides the framework for initial classification, continuous monitoring systems can detect short-term fluctuations that periodic testing might completely overlook [1][2]. By offering real-time data on particle counts, pressure differentials, temperature, and humidity, these systems enable operators to maintain aseptic conditions and address potential contamination risks before they escalate.
Human factors also significantly influence contamination control. Since human activity is the leading source of microbial contamination in cleanrooms [9], aligning monitoring data with personnel movements, gowning protocols, and operational conditions is crucial. Automated systems integrated with Building Management Systems offer timestamped, tamper-proof audit trails - key for meeting regulatory expectations, especially given that over 30% of FDA citations involve Quality Systems [1].
Continuous monitoring also mitigates regulatory risks, as highlighted by the FDA's warning to Optikem International Inc. in June 2024. This case underscored the dangers of relying solely on periodic checks, which allowed critical contamination events to go unnoticed. The result was a determination that the facility was unfit for sterile production, requiring a thorough contamination hazards risk assessment [1].
FAQs
How do I decide what to monitor in my cleanroom first?
To ensure compliance with ISO 14644 and maintain a stable controlled environment for cultivated meat production, it's essential to focus on a few key parameters. These include particle counts, air pressure differentials, temperature, and humidity - all of which play a direct role in maintaining air cleanliness and environmental stability.
It's also important to prioritise monitoring efforts based on areas most at risk of contamination. Factors like personnel movement and material handling can significantly impact cleanliness. Strategically place sampling points in critical zones to gather representative data and ensure effective monitoring.
How often should I sample particles and microbes in ISO Class 5 areas?
Particle sampling in ISO Class 5 areas needs to happen at least every six months to ensure standards are upheld. For microbial testing, the frequency is determined by risk assessments and the monitoring plans already in place. These plans are designed to align with ISO 14644 standards, and it's crucial to review them regularly. This helps maintain the cleanroom's integrity and ensures all regulatory requirements are met.
What should I do when an alert or action limit is breached?
If an alert limit is exceeded, it’s important to step up monitoring, look into possible causes, and record your findings. On the other hand, breaching an action limit requires immediate intervention - this might include stopping operations if necessary, pinpointing the root cause, and taking corrective action. Following these procedures helps ensure compliance with ISO 14644 standards and preserves cleanroom conditions, which are crucial for environments like those used in cultivated meat production.











