Monitoring production spaces for cultivated meat is essential to meet Good Manufacturing Practice (GMP) standards. This ensures food safety and quality by controlling contamination risks like microbes and particles. Key practices include cleanroom classifications (ISO 5–8), air and surface monitoring, and personnel hygiene checks. Facilities must document compliance, follow strict protocols, and use validated systems for data integrity. Regular reviews and trend analysis help maintain control and adapt to evolving standards. Here's what you need to know:
- Cleanroom Standards: ISO 5–8 and GMP Grades A–D guide particle and microbial limits.
- Airborne Monitoring: Laser counters and active/passive microbial sampling are used.
- Surface Monitoring: Contact plates and swabs test for contamination.
- Personnel Hygiene: Glove and garment sampling reduce human contamination risks.
- Documentation: Records, SOPs, and validated systems ensure compliance.
Accurate monitoring supports safety and regulatory adherence, protecting both producers and consumers.
Classification and Routine Environmental Monitoring for GMP Cleanrooms
GMP Requirements for Environmental Monitoring
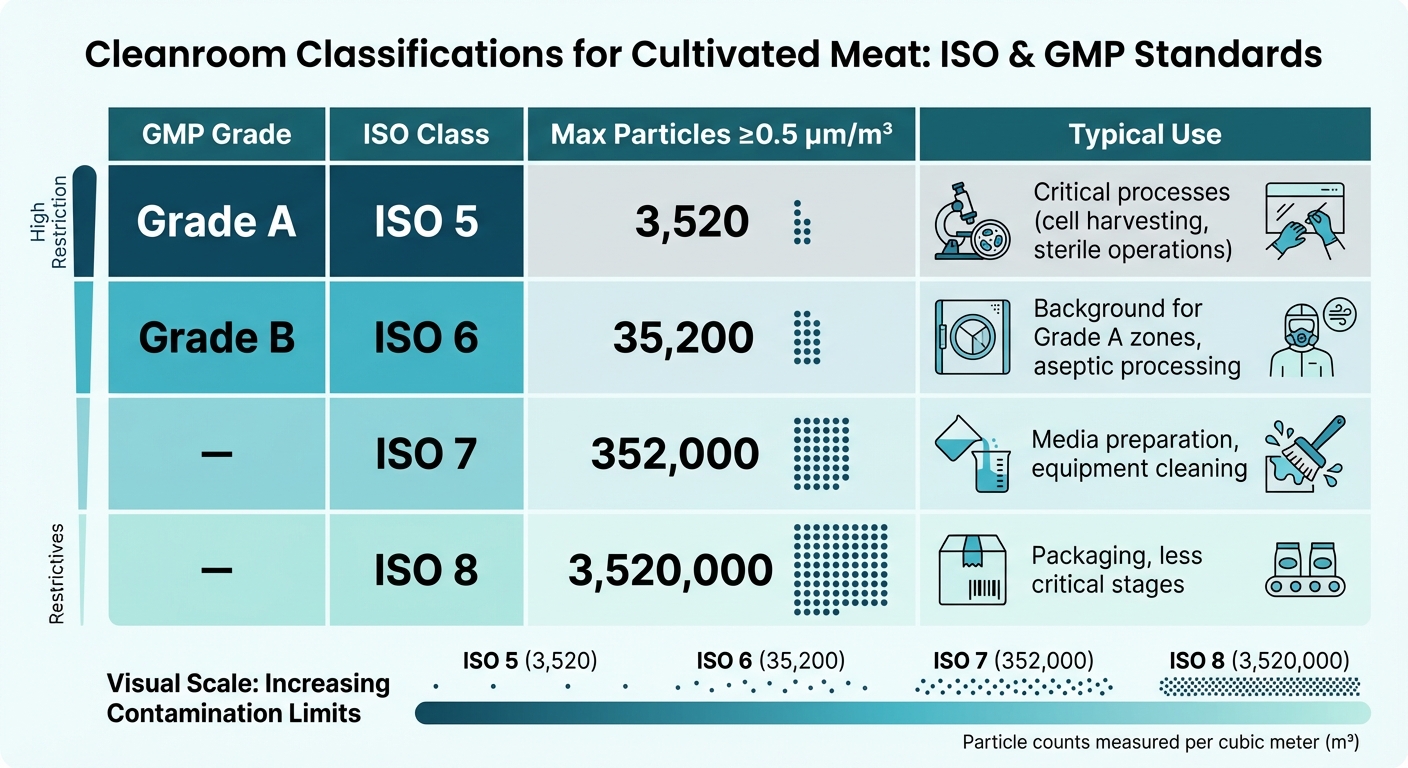
GMP Cleanroom Classifications: ISO Standards and Particle Limits for Cultivated Meat Production
Cleanroom Classifications and Standards
In cultivated meat production, cleanroom classifications follow two primary systems: ISO 14644-1 and GMP Grades A–D. ISO 14644-1 defines cleanroom classes, ranging from ISO 1 (the cleanest, with just 10 particles ≥0.1 µm/m³) to ISO 9. For cultivated meat, ISO 5 through ISO 8 are most commonly employed, depending on the sensitivity of the process.
GMP classifications go beyond particle counts, establishing microbiological limits as well. For example:
- Grade A (ISO 5): Used for critical processes like cell harvesting, where sterility is paramount. It allows a maximum of 3,520 particles ≥0.5 µm/m³.
- Grade B (ISO 6): Serves as the background environment for Grade A zones, commonly used during aseptic processing. It permits up to 35,200 particles ≥0.5 µm/m³.
- ISO 7 and ISO 8: These are suitable for less critical stages, such as media preparation, cleaning equipment, or packaging. They allow up to 352,000 and 3,520,000 particles ≥0.5 µm/m³, respectively.
The choice of classification depends on the contamination risk at each stage of production. Maintaining these environments requires HVAC systems equipped with HEPA or ULPA filters, which ensure particle limits are met. Additional measures like pressure differentials, unidirectional airflow (or non-unidirectional when appropriate), and strict gowning protocols are essential. It's worth noting that human operators are often the largest source of microbial contamination, so personnel procedures must be meticulously managed.
Regulatory Expectations and Documentation
Good Manufacturing Practice (GMP) compliance hinges on validated records that demonstrate cleanrooms consistently meet ISO 14644 particle count and sterility requirements. The EU GMP Annex 1, updated in August 2022, introduced the requirement for a Contamination Control Strategy (CCS). This strategy identifies critical control points and aims to improve contamination detection throughout the facility.
Facilities must keep detailed records of routine monitoring for both airborne particles and microbial contamination, including bacteria and spores. To achieve consistency during compliance audits, it’s essential to implement Standard Operating Procedures (SOPs), comprehensive manuals, and standardised templates. Monitoring systems should be validated under FDA 21 CFR Part 11 and EudraLex Annex 11 to ensure data integrity.
ISO 14644-2 highlights the benefits of continuous monitoring over periodic testing. Continuous systems provide a clearer picture of cleanroom performance and may justify extending intervals between formal reclassifications. However, facilities must maintain detailed records of all environmental data to demonstrate they are operating within contamination control limits. These records not only support compliance but also provide insights into environmental metrics critical for maintaining high standards in production.
Key Metrics for Environmental Monitoring
Adhering to GMP standards is crucial for effective contamination control in cultivated meat production. This section breaks down the essential metrics for validating environmental conditions, ensuring compliance and safety.
Before implementing specific control measures, facilities must establish a solid foundation of prerequisites. These include cleaning protocols, personal hygiene, pest control, and waste management. Once in place, monitoring procedures should be validated, with annual reviews conducted whenever there are changes to equipment, cell lines, or production processes. Keeping a centralised Food Safety Folder to log monitoring results, corrective actions, and calibration checks is a must [1]. Within this framework, the following metrics play a key role in maintaining real-time compliance.
Airborne Particle Counts
Airborne particle counts are measured using laser particle counters, calibrated to ISO 21501 standards. These devices work by detecting scattered light as air passes through a laser beam, categorising particles by size based on the voltage pulses they generate. Proper sampling is essential - particle counter tubing should not exceed 1 metre in length, and bends should have a radius of more than 15 cm to avoid larger particles (≥5.0 µm) dropping out. Isokinetic heads must be positioned to face the airflow direction, or pointed vertically if the airflow is non-unidirectional. Continuous monitoring is required, even beyond the calibration frequency of the counters.
GMP guidelines specify that particle counts should be reported in cumulative mode. For instance, the count for particles ≥0.5 µm includes all particles also counted in the ≥5.0 µm range. Modern Facility Monitoring Systems should employ hot standby architecture to ensure data integrity [3][4].
Viable Microbiological Air Monitoring
Viable air monitoring focuses on identifying living microorganisms that could compromise the production environment. Two main methods are used:
- Active Sampling: A calibrated air sampler impacts a defined air volume onto agar plates, such as Tryptic Soy Agar (for bacteria) or Sabouraud Dextrose Agar (for fungi).
- Passive Sampling: Settling plates capture microbial fallout over time.
Samples on Tryptic Soy Agar are incubated at 30–35°C for at least three days, while those on Sabouraud Dextrose Agar are incubated at 20–25°C for at least seven days. In March 2023, the Sterility Testing Service at the National Institutes of Health Clinical Center introduced a validated microbial control protocol for cellular therapy manufacturing, led by Amanda D. East and Anna F. Lau. This programme combined non-viable particle monitoring, active air sampling, and USP <71> sterility testing with stepwise aseptic practices. Amanda D. East highlighted:
"a well-validated and holistic programme that incorporates robust gowning, cleaning, environmental monitoring, and personnel monitoring measures is critical for minimising the microbial bioburden."
When transferring materials between lower- and higher-classified areas (e.g., ISO 8 to ISO 7), items should be decontaminated with 70% sterile isopropyl alcohol [3].
Surface Cleanliness Monitoring
Surface monitoring methods vary depending on the area being tested. Flat surfaces are typically sampled using contact plates like Count-Tact, while sterile swabs are better suited for irregular surfaces or presence/absence tests. Neutralisers such as lecithinase and Tween 80 should be included in the culture media to counteract residual disinfectants.
For contact plates, apply firm pressure for 5–10 seconds without lateral movement to ensure clear colony formation. Swabbing requires a sterile swab moistened with sterile water, sampling a 5×5 cm area before cleaning the surface with 70% isopropyl alcohol. After sampling, surfaces should be wiped with 70% sterile isopropyl alcohol to remove any residual media. Incubation should be conducted at 32°C (±1.5°C) for 48–72 hours (bacteria) and at 25°C (±1.5°C) for 72 hours (moulds). For sterile filling walls, alert levels are typically set as low as 2 colonies per plate.
As outlined in the MICLAB-045 SOP:
"Alert Levels are accepted levels of contamination that have been derived statistically from 'historical data', i.e. Levels that can be achieved under optimal operating conditions and the GMP guidelines."
If three consecutive monitoring results exceed the alert level - even if they remain below the action level - a Deviation Report should be raised [5].
Personnel Monitoring and Gowning Validation
Personnel are one of the main contamination risks in controlled environments. Monitoring involves gloved hand impressions and contact plate sampling of garments. Letheen Agar is recommended for gloved hand impressions, as it neutralises residual disinfectants. Gowning validation follows a stepwise process when transitioning between ISO 8 and ISO 7 zones. This includes using tacky mats to remove debris from shoes and decontaminating gloves with 70% sterile isopropyl alcohol between gowning steps.
For high-risk tasks, such as work in a Biological Safety Cabinet, sterile sleeves and gloves are recommended. Regular inspections of gowning materials are necessary, and any compromised items should be replaced immediately. Amanda D. East and her colleagues at the National Institutes of Health noted:
"the acceptability criteria for GFS [Gloved Fingertip Sampling] is <1 CFU/plate (i.e., no growth) as per PIC/S 009-16."
In sterile preparation areas, alert limits for gloved hands are typically 3 CFU, with action limits set at 5 CFU. Similar criteria apply to garments.
| Monitoring Type | Sampling Location | Alert Limit (CFU) | Action Limit (CFU) |
|---|---|---|---|
| Personnel | Gloved Hands (Sterile Filling) | <1 | <1 |
| Personnel | Gloved Hands (Sterile Prep) | 3 | 5 |
| Garment | Hood (centre front) | 3 | 5 |
| Garment | Uniform (sleeve/chest) | 3 | 5 |
| Surface | Walls (Sterile Filling) | 2 | 4 (2× Alert) |
sbb-itb-ffee270
Using Environmental Monitoring Data for Compliance
Environmental monitoring data becomes invaluable when it's turned into practical insights that help prevent contamination before it happens. This data forms the foundation for statistical analyses that ensure compliance is maintained consistently.
Statistical Process Control for Trend Analysis
Environmental Monitoring Performance Qualification (EMPQ) plays a key role in confirming that HVAC systems, cleanroom designs, cleaning protocols, and gowning procedures meet microbial and particulate standards. As BioPhorum explains:
"Data from environmental monitoring performance qualification (EMPQ) ensures that the cleanroom environments perform within predefined parameters and provide documented verification that the HVAC system, cleanroom design, cleaning and disinfection program, personnel gowning, material transfer and operation of the equipment are capable of meeting predefined microbial and particulate quality limits." [6]
For new facilities, EMPQ data sets the baseline for determining alert thresholds and action limits. This allows for the early identification of changes in microbial flora or particulate levels, enabling timely corrective actions. These statistical techniques directly support the GMP framework by keeping environmental parameters within validated limits.
To maximise the effectiveness of monitoring, risk-based sampling is essential. When deciding where to place monitoring points, consider factors such as the movement of personnel and materials, as well as the proximity to open products or product-contact surfaces. This approach ensures that resources are directed to areas where contamination risks are highest [2].
Alert and Action Levels by Cleanroom Grade
Alert and action levels vary across cleanroom classifications. Standards like EU GMP Annex 1 typically specify action levels, while alert levels are determined based on a facility’s historical data and validated operational conditions [4]. Repeated breaches of alert levels require investigation and proper documentation, whereas exceeding action levels calls for immediate corrective action.
An effective contamination control strategy relies on integrating environmental monitoring data with broader quality systems. For cultivated meat production, this means using environmental data within Hazard Analysis and Critical Control Points (HACCP) frameworks to address biological, chemical, and physical risks throughout the process [7][8]. Continuous monitoring systems should operate during all production phases, delivering real-time data to ensure environmental conditions stay within specified ranges [4]. When combined with GMP protocols, these measures create a strong foundation for contamination control.
For those working in cultivated meat production, platforms like Cellbase can provide access to essential equipment and materials, helping to establish and maintain effective environmental monitoring systems.
Conclusion
Environmental monitoring isn’t just a box-ticking exercise; it’s the backbone of contamination control in cultivated meat production. As the Food Standards Agency highlights, "HACCP-based procedures for controlling hazards throughout food production will not be effective unless good hygiene practices are also being followed" [1]. To build a robust quality framework, your monitoring systems must seamlessly integrate with established procedures.
By tracking airborne particle counts, conducting surface monitoring, and assessing HVAC performance, you can spot potential breaches before they compromise product safety. This proactive approach is especially critical in cultivated meat production, where challenges like microbial contamination, cell line instability, or toxin build-up can arise.
Reliable monitoring starts with equipment validation and calibration. Particle counters, for instance, need to be calibrated to recognised standards like ISO21501, and all monitoring instruments require regular checks to ensure accurate data. It’s equally important to keep your facility monitoring system separate from broader building management systems, ensuring a clear divide between GxP-critical and non-critical data [4]. This meticulous validation process is key to maintaining operational reliability.
Don’t forget to review your environmental monitoring protocols annually [1]. Regular updates help keep your systems aligned with changing standards. For cultivated meat producers looking for specialised tools, Cellbase offers the equipment and materials needed to set up and maintain effective environmental monitoring systems.
FAQs
What is the difference between ISO 14644-1 cleanroom classifications and GMP Grades A–D?
ISO 14644-1 and GMP Grades A–D serve different purposes when it comes to cleanroom classification, and their systems reflect this difference. ISO 14644-1 uses a numerical scale ranging from 1 to 9, where ISO 1 represents the cleanest environment, based on air particle concentration. On the other hand, GMP Grades A–D use a letter-based system. Grade A signifies the highest cleanliness level required for critical operations, while Grades B through D apply to areas with progressively lower cleanliness requirements.
The ISO standards focus on measuring and limiting airborne particle counts, offering a globally recognised framework for cleanroom classification. GMP grades, however, are designed with regulatory compliance in mind, setting specific contamination thresholds for manufacturing processes. This is particularly important in sectors like pharmaceuticals and cultivated meat production. Both systems aim to control contamination, but GMP grades are more directly tied to meeting strict regulatory and production standards.
How does continuous environmental monitoring support GMP compliance?
Continuous monitoring of environmental conditions plays a key role in upholding GMP compliance. It enables real-time detection of changes in crucial factors such as air quality, surface cleanliness, and equipment performance. This immediate awareness helps reduce contamination risks and ensures that cultivated meat production remains consistent in quality and safety.
With timely data on environmental parameters, facilities can act quickly to address issues, meeting strict regulatory standards while improving production processes. This approach not only supports reliable, high-quality output but also reinforces consumer confidence in the product.
Why is personnel hygiene important for preventing contamination in cleanrooms used for cultivated meat production?
Maintaining personal hygiene is a cornerstone of preventing contamination in cleanrooms, which are crucial for producing cultivated meat. Simple but essential practices - like meticulous handwashing, wearing appropriate protective gear, and following aseptic techniques - play a key role in minimising the introduction of microbes or particles into these tightly controlled spaces.
These hygiene protocols are part of a larger environmental monitoring system that involves regularly testing air, surfaces, and even personnel. By upholding stringent hygiene standards, cleanrooms can remain controlled and compliant with GMP regulations, protecting both the quality and safety of cultivated meat products.











